- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记4.3.3 Properties of Covalent Compounds
Properties of Covalent Compounds
- The physical properties of molecular covalent compounds are largely influenced by their intermolecular forces
- If you know the type of intermolecular forces present you can predict the physical properties like melting and boiling point, solubility, and conductivity
Melting and boiling point
- When covalent molecular substances change state you are overcoming the intermolecular forces
- The stronger the forces the more energy need to break the attraction
- Intermolecular forces are much weaker than covalent bonds, so many covalent substances are liquid or gases at room temperature
- Substance with a low melting and boiling point are said to be very volatile
- The strength of the intermolecular forces increases with
- the size of the molecule
- the increase in the polarity of the molecule
- Drawing the structure of the molecule helps identify and rank molecules according to boiling point as the following example shows:
Worked Example
Place these three molecules in the correct order from lowest to highest boiling point and explain your reasoning:
CH3CH2CH2OH CH3COCH3 CH3CH2CH2CH3
Answer:
Step 1: The first thing to do is find the approximate relative molecular mass:
CH3CH2CH2OH = 60
CH3COCH3 = 58
CH3CH2CH2CH3 = 58
This tells you the molecules are approximately the same size so the dispersion forces will be similar
Step 2: Draw the structures of the molecules and identify the intermolecular forces present
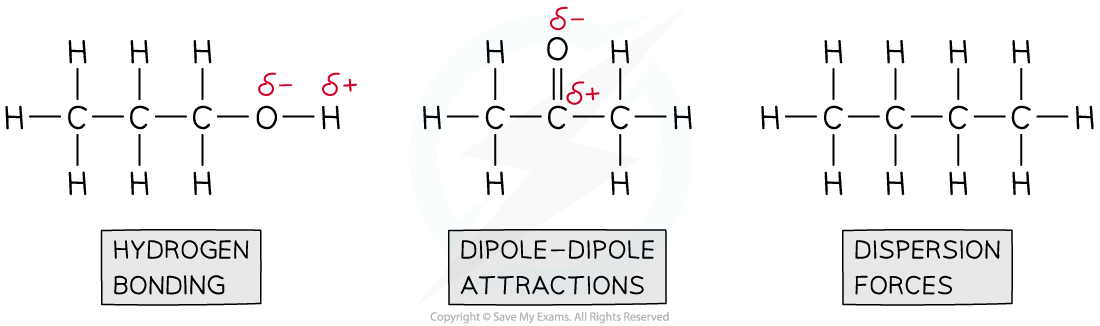
So, the order of boiling from lowest to highest is:
CH3CH2CH2CH3 ˂ CH3COCH3 ˂ CH3CH2CH2OH
Solubility
- The general principle is that 'like dissolves like' so non-polar substances mostly dissolve in non-polar solvents, like hydrocarbons and they form dispersion forces between the solvent and the solute
- Polar covalent substances generally dissolve in polar solvents as a result of dipole-dipole interactions or the formation of hydrogen bonds between the solute and the solvent
- A good example of this is seen in organic molecules such as alcohols and water:
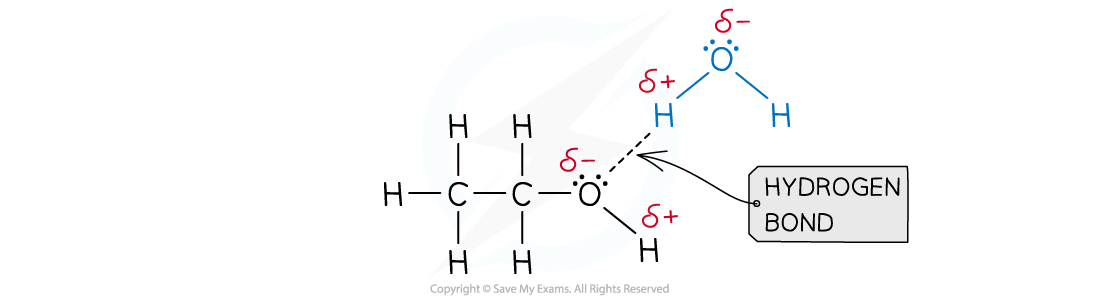 Hydrogen bonds form between ethanol and water
Hydrogen bonds form between ethanol and water
- As covalent molecules become larger their solubility can decrease as the polar part of the molecule is only a smaller part of the overall structure
- This effect is seen in alcohols for example where ethanol, C2H5OH, is readily soluble but hexanol, C6H13OH, is not
- Polar covalent substances are unable to dissolve well in non-polar solvents as their dipole-ipole attractions are unable to interact well with the solvent
- Giant covalent substances generally don't dissolve in any solvents as the energy needed to overcome the strong covalent bonds in the lattice structures is too great
Conductivity
- As covalent substances do not contain any freely moving charged particles they are unable to conduct electricity in either the solid or liquid state
- However, under certain conditions some polar covalent molecules can ionise and will conduct electricity
- Some giant covalent structures are capable of conducting electricity due to delocalised electrons, as seen in Section 4.1.11 Giant Covalent Structures, but they are exceptions to the general rule
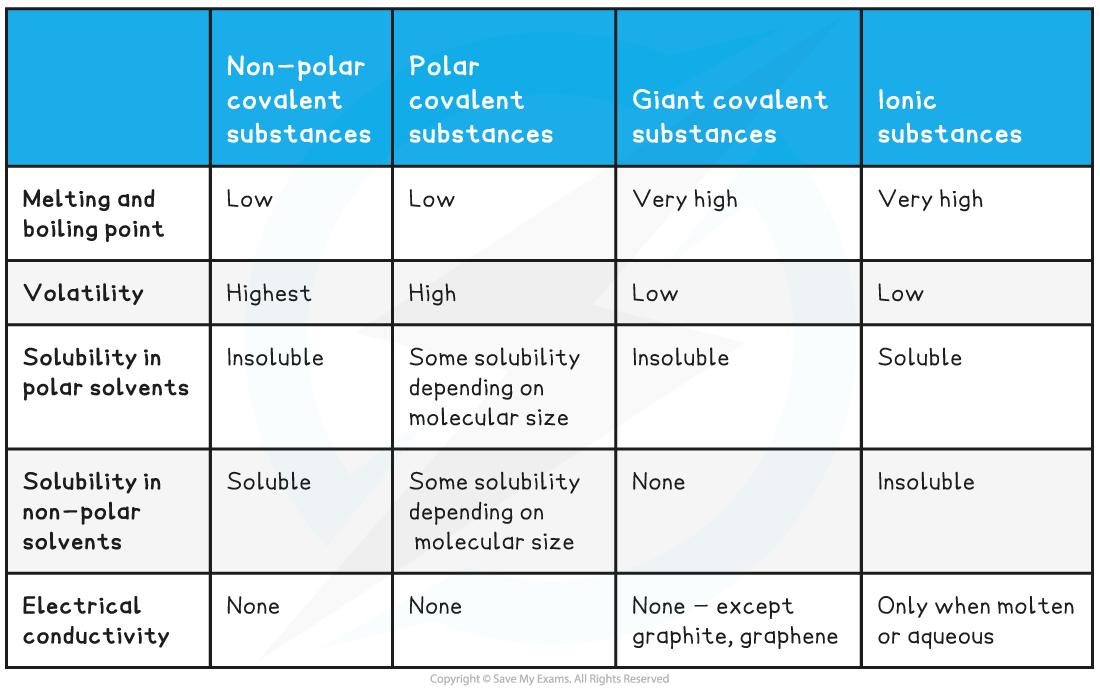
Comparing the Properties of Covalent Compounds Table
Worked Example
Compound X has the following properties: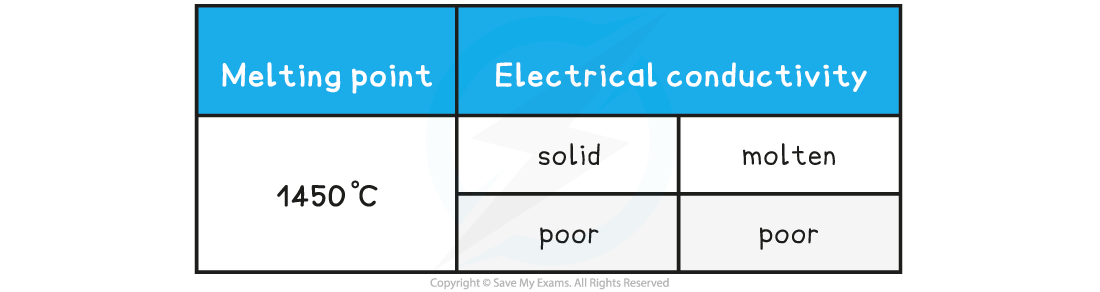 What is the most probable structure of X?
What is the most probable structure of X?
A. Network covalent
B. Polar covalent molecule
C. Ionic lattice
D. Metallic lattice
Answer:
The correct option is A
-
- A high melting point is characteristic of a giant structure, which could be metallic, ionic or covalent
- The poor conductivity as a liquid and solid would match a giant covalent or network covalent structure
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








