- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记4.2.4 Molecular Polarity
Molecular Polarity
Assigning polarity to molecules
- There is a difference between bond polarity and molecular polarity
- To determine whether a molecule is polar, the following things have to be taken into consideration:
- The polarity of each bond
- How the bonds are arranged in the molecule
- Some molecules have polar bonds but are overall not polar because the polar bonds in the molecule are arranged in such way that the individual dipole moments cancel each other out
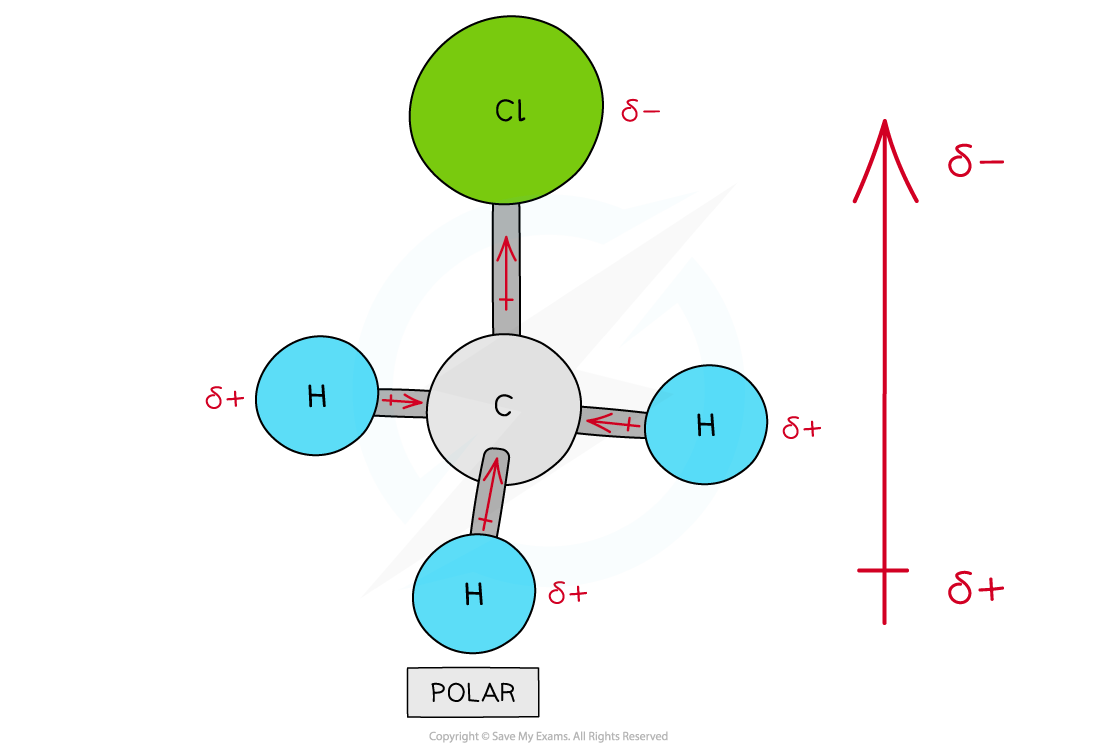
There are four polar covalent bonds in CH3Cl which do not cancel each other out causing CH3Cl to be a polar molecule; the overall dipole is towards the electronegative chlorine atom
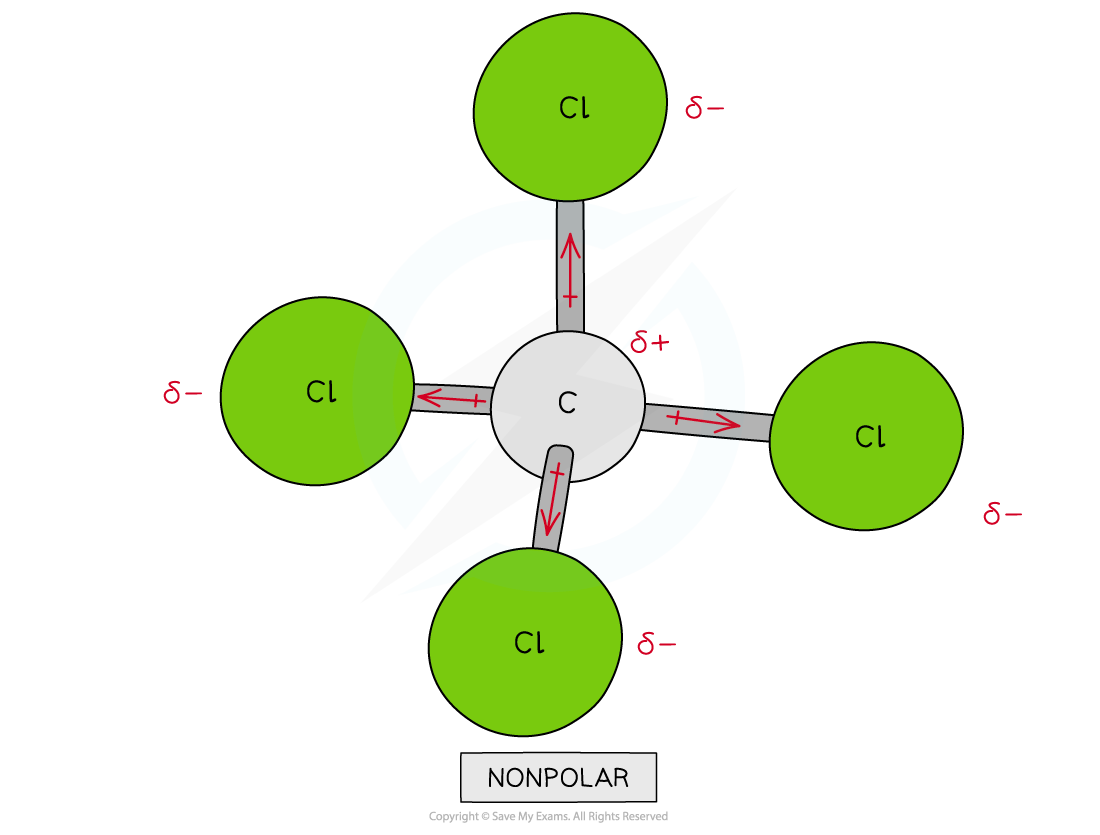
Though CCl4 has four polar covalent bonds, the individual dipole moments cancel each other out causing CCl4 to be a nonpolar molecule
- Further examples of molecules with no net dipole:
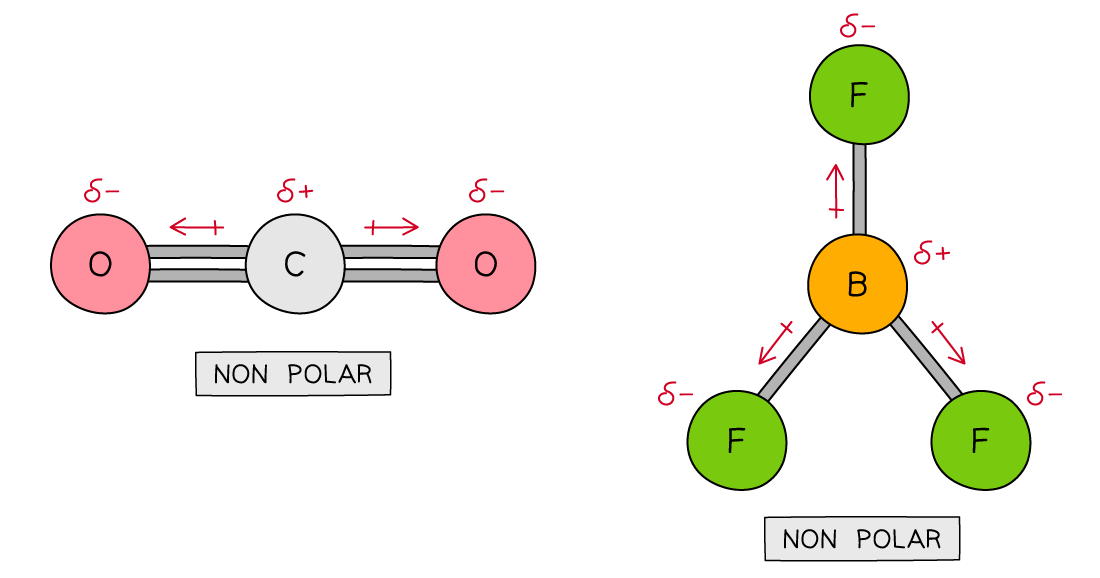
Carbon dioxide and boron trifluoride have polar bonds but no net dipole
- Try your hand at this polarity question:
Worked Example
Which molecule is non-polar?
A. NH3
B. CO
C. SO2
D. AlBr3
Answer:
The correct option is D.
-
- The shapes and polarity of the molecules are as follows:
 Although the Al-Br bonds are polar, the trigonal planar molecule is symmetrical so the dipoles cancel out leaving a non-polar molecule
Although the Al-Br bonds are polar, the trigonal planar molecule is symmetrical so the dipoles cancel out leaving a non-polar molecule
Exam Tip
One of the clues about molecular polarity is to look at the symmetry of the moleculeMolecules which are symmetrical are unlikely to be polar
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








