- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记3.2.3 Periodic Trends: Group 17 - The Halogens
Halogens
The halogens
- These are the group 17 non-metals that are poisonous and include fluorine, chlorine, bromine, iodine and astatine
- Halogens are diatomic, meaning they form molecules of two atoms
- All halogens have seven electrons in their outer shell
- They form halide ions by gaining one more electron to complete their outer shells
Colours and States at Room Temperature
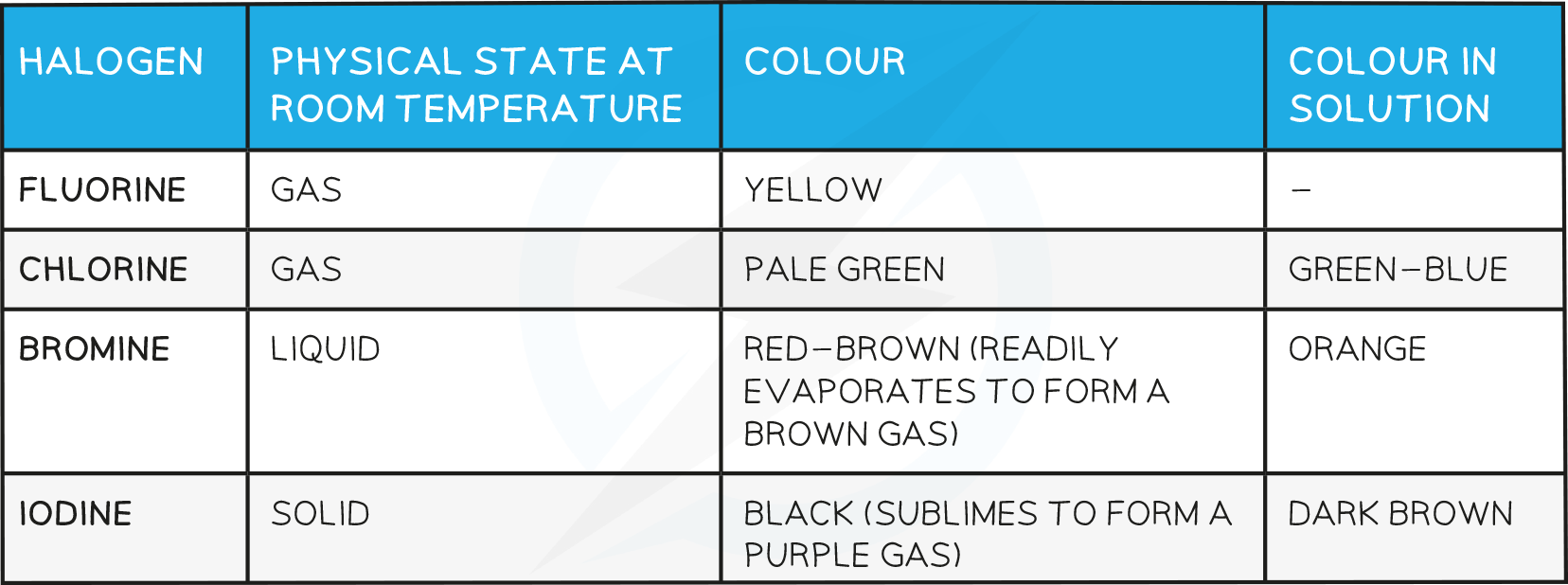
Trends in physical properties of the halogens
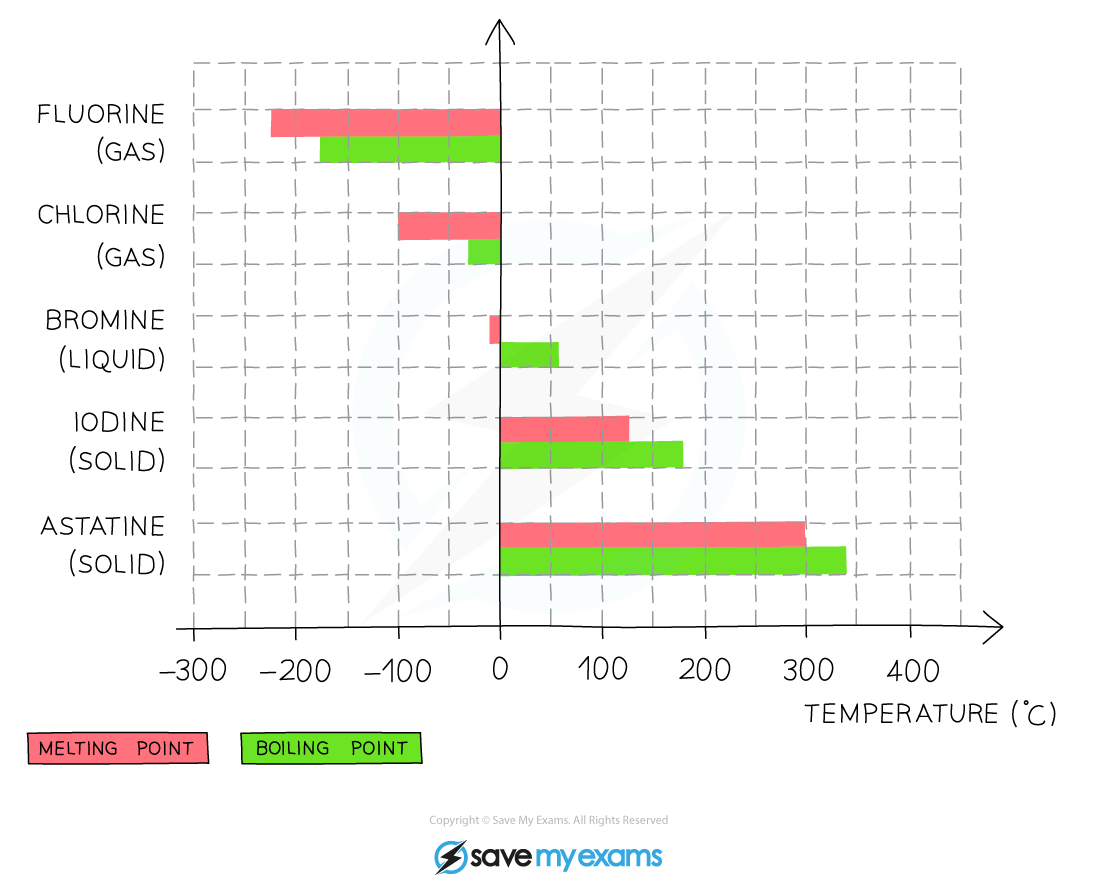
Melting point
- The density and melting and boiling points of the halogens increase as you go down the group
Explaining the trend in reactivity in Group VII
- Reactivity of group 17 non-metals decreases as you go down the group
- The halogens electron configurations all end in ns2np5
- Each outer shell contains seven electrons and when they react, they will need to gain one outer electron to get a full outer shell of electrons
- Going down the group, the electron affinity decreases and the atomic radius increases
- As you go down group 17, the number of shells of electrons increases so shielding also increases
- This means that the outer electrons are further from the nucleus so there are weaker electrostatic forces of attraction that attract the extra electron needed
- The electron is attracted less readily, so the lower down the element is in Group 17 the less reactive it is
Reaction of the halogens with halide ions in displacement reactions
- A halogen displacement occurs when a more reactive halogen displaces a less reactive halogen from an aqueous solution of its halide
- The reactivity of group 17 non-metals increases as you move up the group
- Out of the 3 halogens, chlorine, bromine and iodine, chlorine is the most reactive and iodine is the least reactive
Aqueous Solution Colour of Halogens

Halogen displacement reactions
Chlorine and bromine
- If you add chlorine solution to colourless potassium bromide solution, the solution becomes orange as bromine is formed
- Chlorine is above bromine in group 17 so it is more reactive
- Chlorine will therefore displace bromine from an aqueous solution of a metal bromide
2KBr (aq) + Cl2 (aq) → 2KCl (aq) + Br2(aq)
potassium bromide + chlorine → potassium chloride + bromine
Bromine and iodine
- Bromine is above iodine in group 17 so it is more reactive
- Bromine will therefore displace iodine from an aqueous solution of a metal iodide
Br2 (l) + 2NaI (aq) → 2NaBr (aq) + I2 (aq)
bromine + sodium Iodide → sodium bromide + iodine
Test yourself on halogen displacements:
Worked Example
Which of the statements below are correct?
I. potassium chloride solution will react with fluorine to form chlorine.
II. sodium chloride solution will react with iodine to form chlorine.
III. lithium iodide solution will react with bromine to form iodine.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answer:
The correct option is B.
-
- Fluorine will displace chlorine as it is higher up in the group and bromine will displace iodine for the same reason.
- Iodine is below chlorine so cannot displace chlorine from sodium chloride
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









