- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记3.1.4 Periodic Trends: Physical - Electron Affinity
Electron Affinity
- When atoms gain electrons they become negative ions or anions
- Electron affinity (EA) can be thought of as the opposite process of ionisation energy and is defined as
- The amount of energy released when one mole of electrons is gained by one mole of atoms of an element in the gaseous state to form one mole of gaseous ions
- Electron affinities are measured under standard conditions which are 298 K and 100 kPa
- The units of EA are kilojoules per mole (kJ mol-1)
- The first electron affinity is always exothermic
- E.g. the first electron affinity of chlorine is:
Cl (g) + e- → Cl- (g) ∆H = - 349 kJ mol-1
- However, the second electron affinity can be an endothermic process
O- (g) + e- → O2- (g) ∆H = + 753 kJ mol-1
- This is due to the fact that you are overcoming repulsion between the electron and a negative ion, so energy is required making the process endothermic overall
Trends in electron affinity
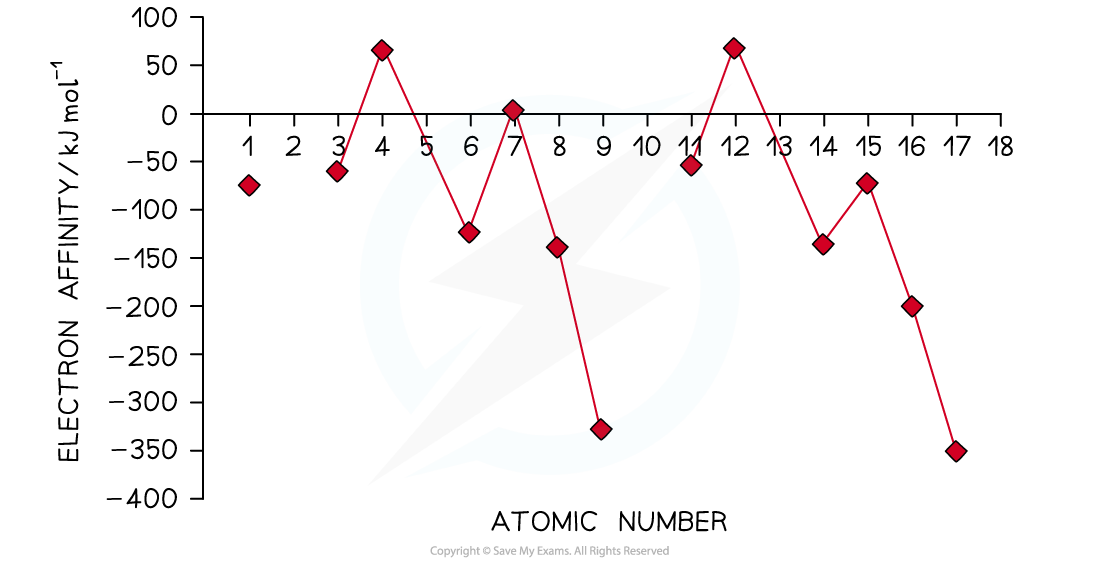
Electron affinities across a period
- Electron affinities show periodicity
- The pattern is very similar to ionisation energies, except that it is inverted and the minimum points are displaced one element to the right
- As might be expected, the most exothermic electron affinities are for group 17 elements which also have the highest electronegativities
- The strongest pull on electrons correlates with the greater amount of energy released when negative ions are formed
- Noble gases do not form negative ions, so they don't appear in this chart
- The electron affinities reach a peak for group 2 and group 5 elements
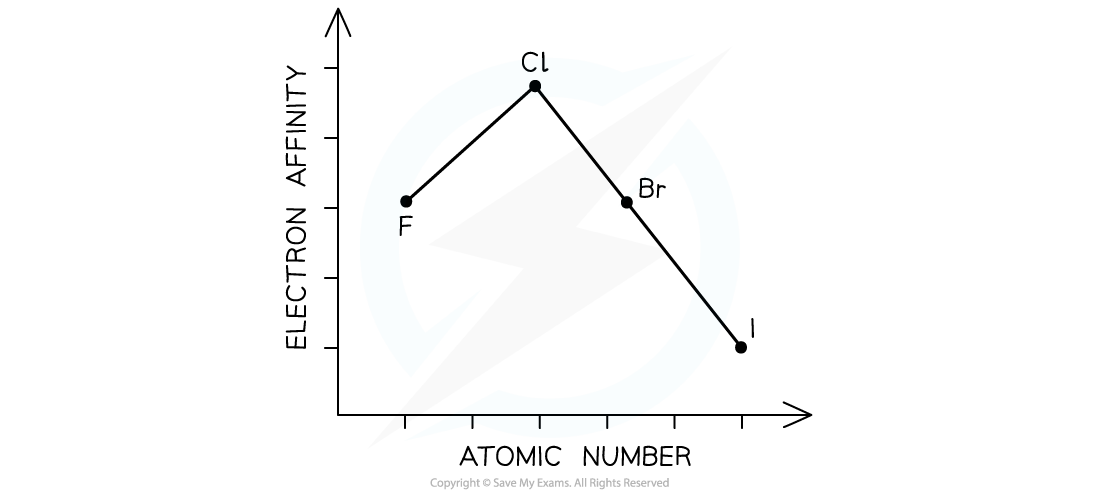
Electron affinities down a group
- Electron affinities generally decrease down a group
- As the atoms become larger the attraction for an additional electron is less, since the effective nuclear charge is reduced due to increased shielding
- Electron affinity become less exothermic going down the group
- An exception to this is fluorine whose electron affinity is smaller than expected
- This is because fluorine is such a small atom and an additional electron in the 2p subshell experiences considerable repulsion with the other valence electrons
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








