- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记2.1.1 The Nuclear Atom
Mass & Charge Distribution
- The mass of an atom is concentrated in the nucleus, because the nucleus contains the heaviest subatomic particles (the neutrons and protons)
- The mass of the electron is negligible
- The nucleus is also positively charged due to the protons
- Electrons orbit the nucleus of the atom, contributing very little to its overall mass, but creating a ‘cloud’ of negative charge
- The electrostatic attraction between the positive nucleus and negatively charged electrons orbiting around it is what holds an atom together
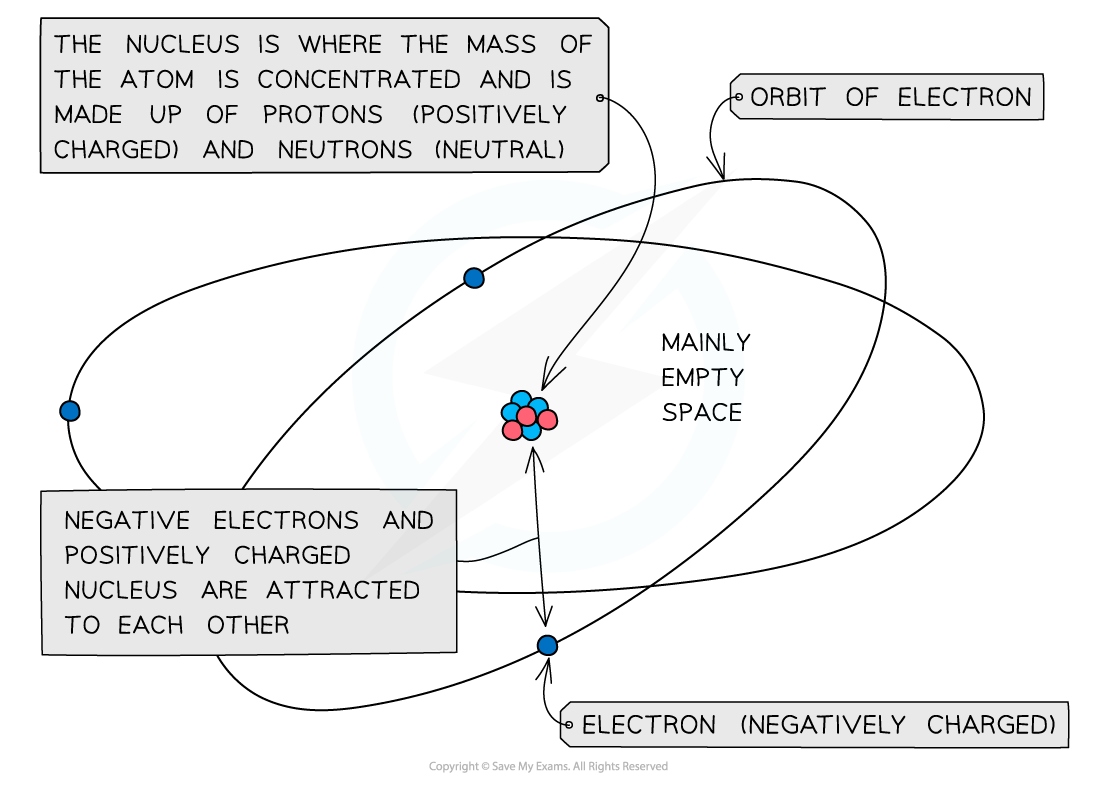
The mass of the atom is concentrated in the positively charged nucleus which is attracted to the negatively charged electrons orbiting around it
Types of Subatomic Particles
- The protons, neutrons and electrons that an atom is made up of are called subatomic particles
- These subatomic particles are so small that it is not practical to measure their masses and charges using conventional units (such as grams or coulombs)
- Instead, their masses and charges are compared to each other, and so are called ‘relative atomic masses’ and ‘relative atomic charges’
- These are not actual charges and masses, but rather charges and masses of particles relative to each other
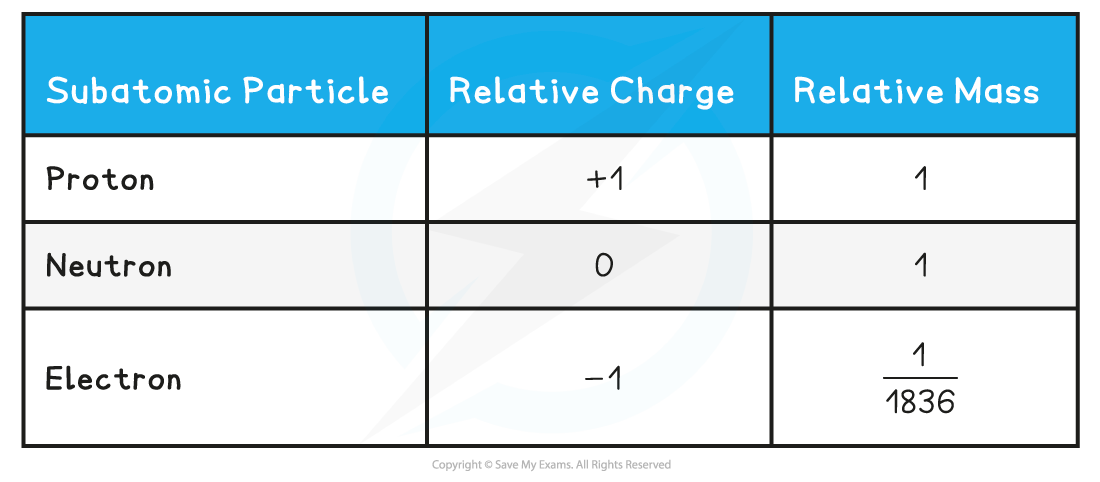
- Protons and neutrons have a very similar mass, so each is assigned a relative mass of 1
- Electrons are 1836 times smaller than a proton and neutron, and so their mass is often described as being negligible
- The relative mass and charge of the subatomic particles are:
Relative Mass & Charge of Subatomic Particles Table
Exam Tip
You can see from the table how the relative mass of an electron is almost negligibleThe charge of a single electron is -1.602189 x 10-19 coulombs, whereas the charge of a proton is +1.602189 x 10-19 coulombs. However, relative to each other, their charges are -1 and +1 respectively. This information can also been found in the IB Data Booklet
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








