- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记1.2.8 Concentration Calculations
Concentration Calculations
Step by step
- Concentration calculations involve bringing together the skills and knowledge you have acquired previously and applying them to problem solving
- You should be able to easily convert between moles, mass, concentrations and volumes ( of solutions and gases)
- The four steps involved in problem solving are:
- write the balanced equation for the reaction
- determine the mass/ moles/ concentration/ volume of the of the substance(s) you know about
- use the balanced equation to deduce the mole ratios of the substances present
- calculate the mass/ moles/ concentration/ volume of the of the unknown substance(s)
Worked Example
25.0 cm3 of 0.050 mol dm-3 sodium carbonate was completely neutralised by 20.0 cm3 of dilute hydrochloric acid.Calculate the concentration in mol dm-3 of the hydrochloric acid.
Answer:
Step 1: Write the balanced equation for the reaction
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
Step 2: Determine the moles of the known substance, in this case sodium carbonate. Don't forget to divide the volume by 1000 to convert cm3 to dm3
moles = volume x concentration
amount (Na2CO3) = 0.0250 dm3 x 0.050 mol dm-3 = 0.00125 mol
Step 3: Use the balanced equation to deduce the mole ratio of sodium carbonate to hydrochloric acid:
1 mol of Na2CO3 reacts with 2 mol of HCl, so the mole ratio is 1 : 2
Therefore 0.00125 moles of Na2CO3 react with 0.00250 moles of HCl
Step 4: Calculate the concentration of the unknown substance, hydrochloric acid
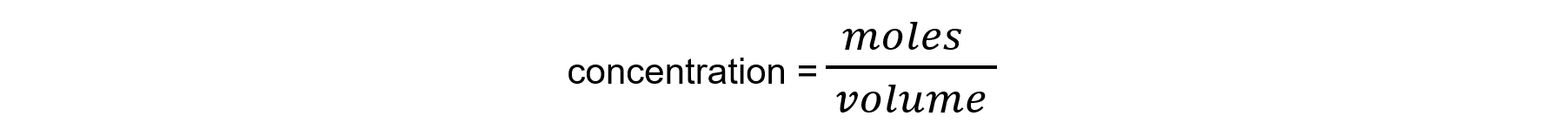
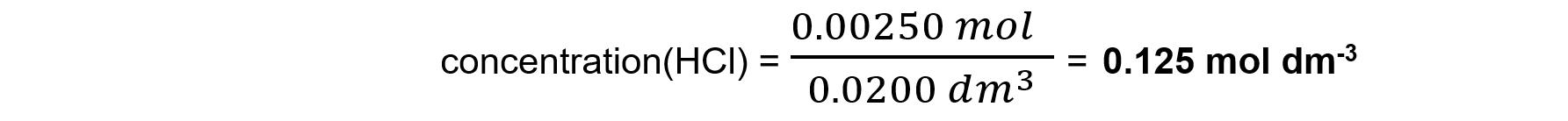
Worked Example
Calculate the volume of hydrochloric acid of concentration 1.0 mol dm-3 that is required to react completely with 2.5 g of calcium carbonate.
Answer:
Step 1: Write the balanced equation for the reaction
CaCO3 + 2HCl → CaCl2 + H2O + CO2
Step 2: Determine the moles of the known substance, calcium carbonate
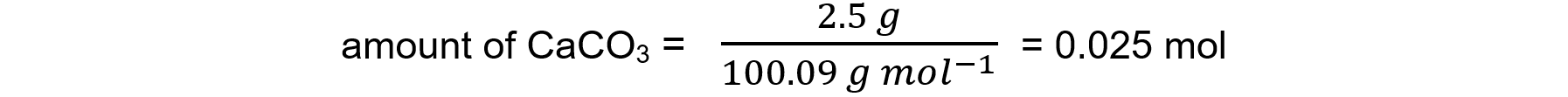 Step 3: Use the balanced equation to deduce the mole ratio of calcium carbonate to hydrochloric acid:
Step 3: Use the balanced equation to deduce the mole ratio of calcium carbonate to hydrochloric acid:
1 mol of CaCO3 requires 2 mol of HCl
So 0.025 mol of CaCO3 requires 0.050 mol of HCl
Step 4: Calculate the volume of HCl required
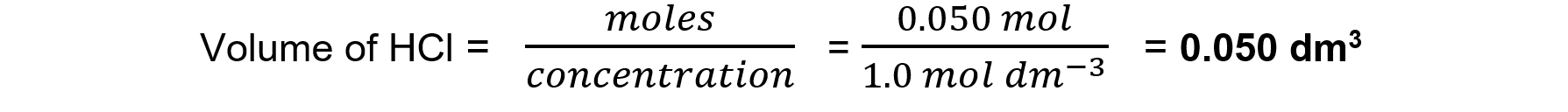
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








