- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记1.2.7 Standard Solutions
Concentrations of Solutions
Standard solutions
- Chemists routinely prepare solutions needed for analysis, whose concentrations are known precisely
- These solutions are termed standard solutions
- They are made as accurately and precisely as possible using three decimal place balances and volumetric flasks to reduce the impact of measurement uncertainties
- The steps are:
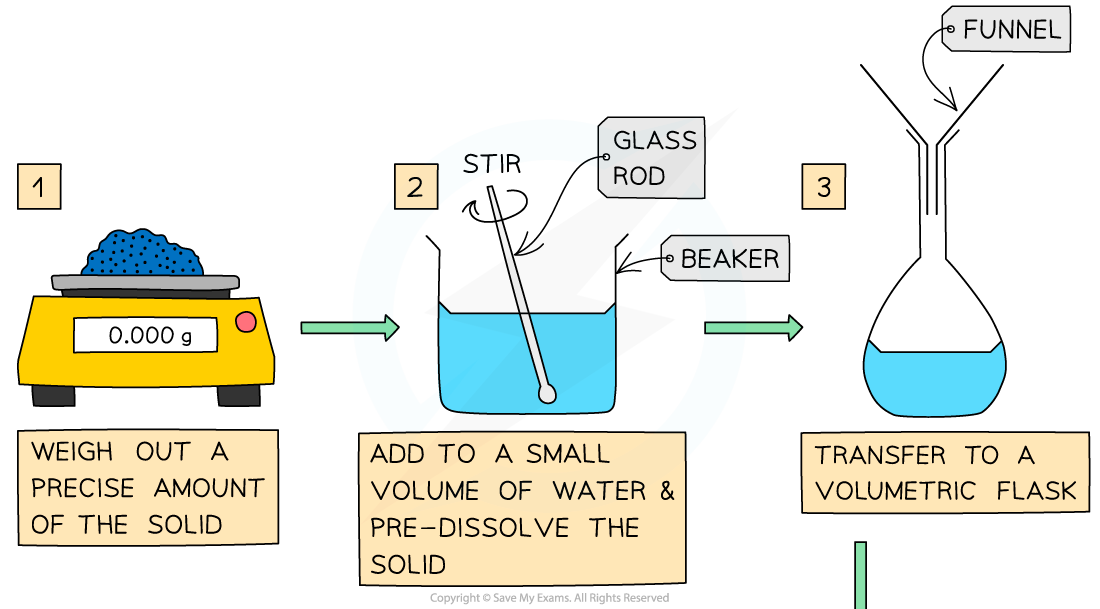
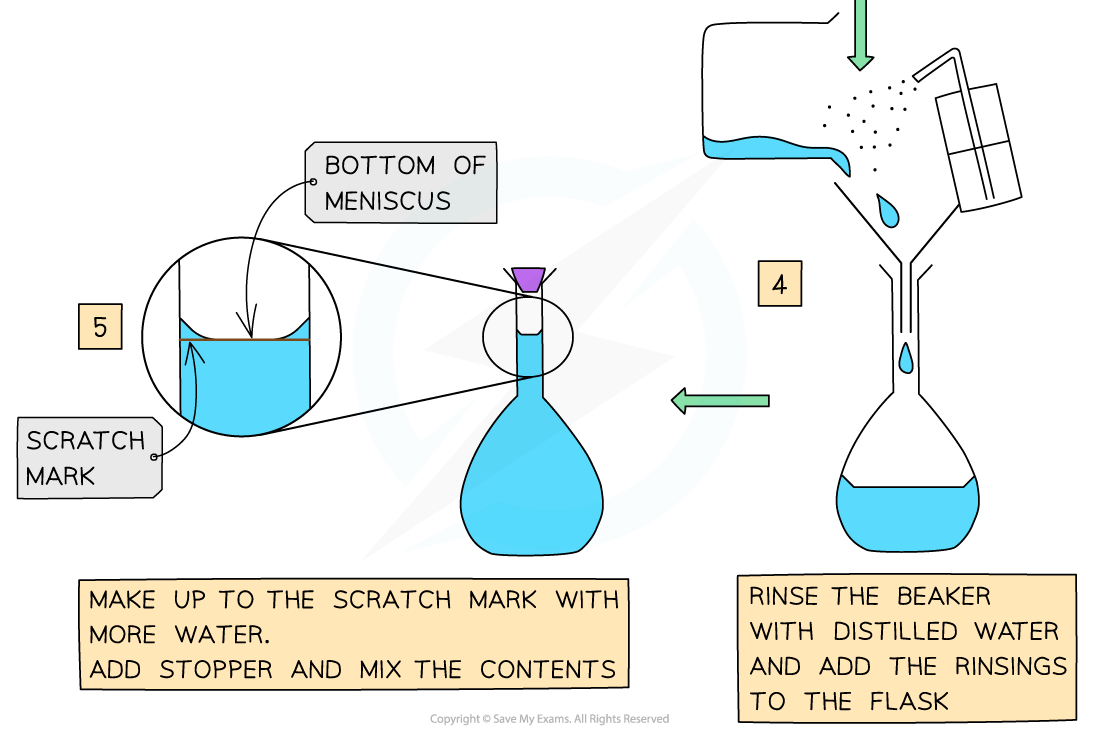
Volumes & concentrations of solutions
- The concentration of a solution is the amount of solute dissolved in a solvent to make 1 dm3 of solution
- The solute is the substance that dissolves in a solvent to form a solution
- The solvent is often water
- A concentrated solution is a solution that has a high concentration of solute
- A dilute solution is a solution with a low concentration of solute
- Concentration is usually expressed in one of three ways:
- moles per unit volume
- mass per unit volume
- parts per million
Moles per unit volume
- The formula for expressing concentration using moles is:
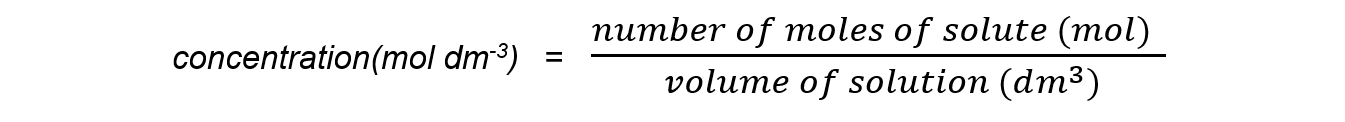
- You must make sure you change cm3 to dm3 (by dividing by 1000)
- The relationships can be expressed using this formula triangle:
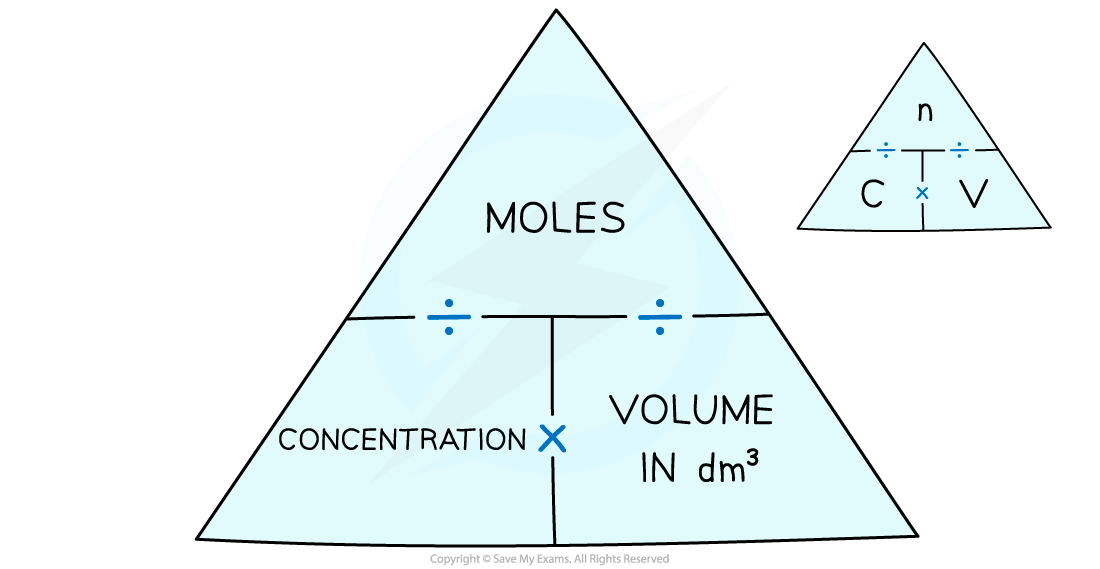
To use the concentration formula triangle cover the one you want to find out about with your finger and follow the instructions
Worked Example
Calculate the mass of sodium hydroxide, NaOH, required to prepare 250 cm3 of a 0.200 mol dm-3 solution
Answer:
Step 1: Use the formula triangle to find the number of moles of NaOH needed
number of moles = concentration (mol dm-3) x volume (dm3)
n = 0.200 mol dm-3 x 0.250 dm3
n = 0.0500 mol
Step 2: Find the molar mass of NaOH
M = 22.99 + 16.00 + 1.01 = 40.00 g mol-1
Step 3: Calculate the mass required
mass = moles x molar mass
mass = 0.0500 mol x 40.00 g mol-1 = 2.00 g
Mass per unit volume
- Sometimes it is more convenient to express concentration in terms of mass per unit volume
- The formula is:
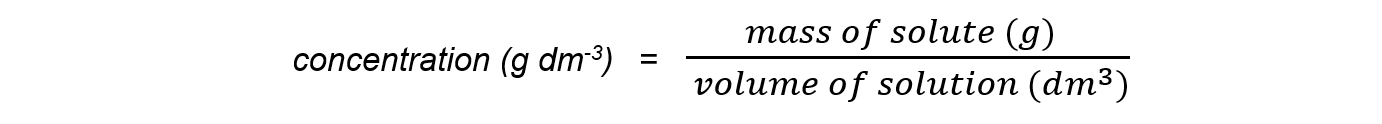
- To change a concentration from mol dm-3 to g dm-3
- Multiply the moles of solute by its molar mass
mass of solute (g) = number of moles (mol) x molar mass (g mol-1)
Parts per million
- When expressing extremely low concentrations a unit that can be used is parts per million or ppm
- This is useful when giving the concentration of a pollutant in water or the air when the absolute amount is tiny compared the the volume of water or air
- 1 ppm is defined as
- A mass of 1 mg dissolved in 1 dm3 of water
- Since 1 dm3 weighs 1 kg we can also say it is
- A mass of 1 mg dissolved in 1 kg of water, or 10-3 g in 103 g which is the same as saying the concentration is 1 in 106 or 1 in a million
Worked Example
The concentration of chlorine in a swimming pool should between between 1 and 3 ppm. Calculate the maximum mass, in kg, of chlorine that should be present in an olympic swimming pool of size 2.5 million litres.
Answer:
Step 1: calculate the total mass in mg assuming 3ppm(1 litre is the same as 1 dm3)
3 x 2.5 x 106 = 7.5 x 106 mg
Step 2: convert the mass into kilograms (1 mg = 10-6 kg)
7.5 x 106 x 10-6 kg = 7.5 kg
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









