- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Biology: SL复习笔记4.2.1 Carbon Cycle: Carbon Dioxide
Carbon Fixation
- Carbon is present in the atmosphere in the form of carbon dioxide gas
- Carbon is taken out of the atmosphere by plants to be used in photosynthesis
- Plants are autotrophs
- Autotrophs use light energy to convert carbon dioxide from the environment into carbon compounds, such as:
- Carbohydrates
- Lipids
- Amino acids
- This reduces the amount of carbon present in the atmosphere and stores it in the tissues of plants
Carbon Dioxide in Solution
- Where the atmosphere comes into contact with bodies of water, carbon dioxide in the atmosphere can dissolve in water
- The oceans are thought to store significantly more carbon than the atmosphere
- Some of the carbon in aquatic systems is in the form of dissolved carbon dioxide
- Some dissolved carbon dioxide reacts with water to form carbonic acid, (H2CO3), which then dissociates to produce hydrogen carbonate ions (HCO3-), and hydrogen (H+) ions
- H+ ions in the water cause it to become more acidic; as more carbon dioxide dissolves in the oceans, the pH of the oceans decreases and this can cause problems for some marine organisms
- Aquatic producers (such as aquatic plants and phytoplankton) can absorb dissolved carbon dioxide and hydrogen carbonate ions for photosynthesis, using light energy from the sun to convert this carbon into other carbon compounds such as carbohydrates
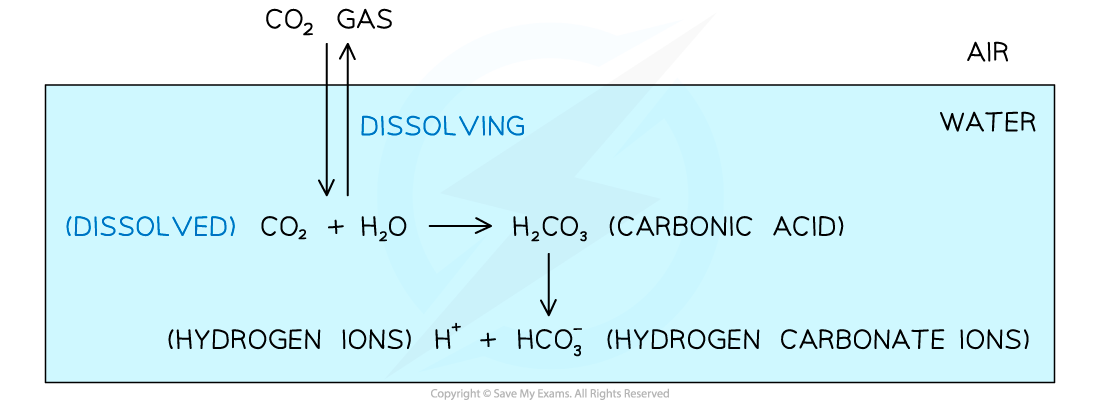
Carbon is present in water in the form of dissolved carbon dioxide and hydrogen carbonate ions
Carbon Dioxide & Coral Reefs
- The impact of increasing carbon dioxide levels on the atmosphere is well known; it is a greenhouse gas and therefore increases the warming of the atmosphere as its atmospheric concentration increases
- The impact of increasing carbon dioxide levels on the oceans are less well-understood by the public, but could be significant for ocean biodiversity because of the effect of carbon dioxide on ocean chemistry
- Huge amounts of carbon dioxide are dissolved by the oceans, and much of the dissolved carbon dioxide reacts with seawater to form carbonic acid (H2CO3)
CO2 + H2O → H2CO3
-
- Carbonic acid then dissociates to form hydrogen ions (H+) and hydrogen carbonate ions (HCO3-)
H2CO3 → H+ + HCO3-
-
- Hydrogen carbonate ions can then dissociate again to form more hydrogen ions and carbonate ions (CO32-)
HCO3- → H+ + CO32-
- Provided that this series of reactions takes place at the appropriate rate, the oceans remain slightly alkaline, and there is a steady supply of carbonate ions for organisms that need them
- Many marine organisms need carbonate ions in order to secrete calcium carbonate for the building of the hard parts of their bodies
- Molluscs such as mussels and clams build their shells from calcium carbonate
- Coral is made up of many tiny organisms called coral polyps which secrete hard exoskeletons built from calcium carbonate; these exoskeletons form the complex structures of corals which are a key part of coral reef ecosystems
- Many marine organisms need carbonate ions in order to secrete calcium carbonate for the building of the hard parts of their bodies
- However, as atmospheric carbon dioxide levels increase, so too does the amount of carbon dioxide that dissolves in the oceans
- As more carbon dioxide dissolves, more carbonic acid forms and dissociates, and more hydrogen carbonate ions form and dissociate, the end result of which is increasing numbers of hydrogen ions in a seawater solution
- Increasing concentrations of hydrogen ions in solution cause that solution to become more acidic; in this case the process is known as ocean acidification
- Note that the oceans are still alkaline, but the pH has decreased, so they are closer to neutral
- There are significant consequences to ocean acidification
- The calcium carbonate exoskeletons of, e.g. corals, can be weakened and even dissolve
- The reaction during which hydrogen carbonate ions dissociate to form hydrogen ions and carbonate ions reverses to buffer the increasing number of hydrogen ions, reducing the availability of carbonate ions for the building of hard exoskeletons
H+ + CO32- → HCO3-
- When the effects of ocean acidification are combined with coral bleaching that results from warming oceans, the consequences for coral reefs could be very serious
- With coral reefs thought to be the most diverse ecosystem we know, this could be bad news for ocean biodiversity
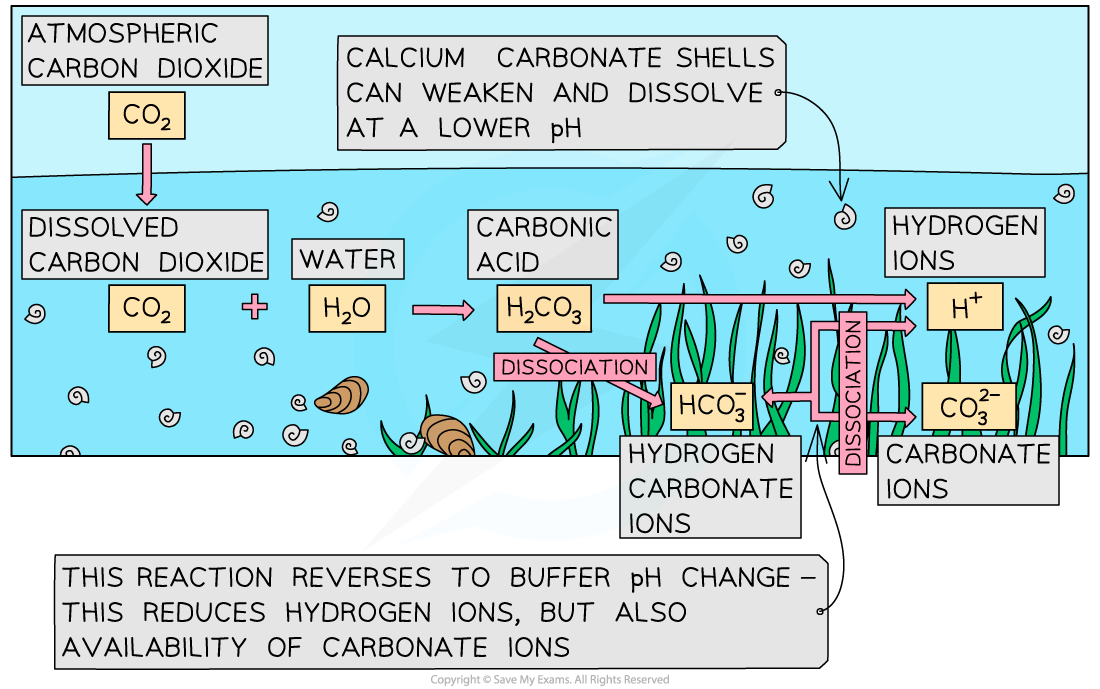
Increased atmospheric carbon dioxide increases the number of hydrogen ions in seawater, and reduces the availability of carbonate ions
Exam Tip
Note that while ocean acidification shares the same cause as global warming (increased atmospheric carbon dioxide), it is not a direct result of global warming.
Carbon Dioxide Absorption
- Autotrophs absorb carbon dioxide from their surroundings (either air or water) before converting it into carbon compounds in their tissues
- This absorption takes place by diffusion into the leaves of plants
- Carbon dioxide diffuses down its concentration gradient from a region of high concentration (outside the leaves) to a region of low concentration (inside leaves)
- The cells inside leaves use carbon dioxide in photosynthesis so the concentration is always low inside the photosynthesising cells, maintaining the concentration gradient
- This diffusion takes place through the stomata of land plants, and directly into the cells of aquatic plants
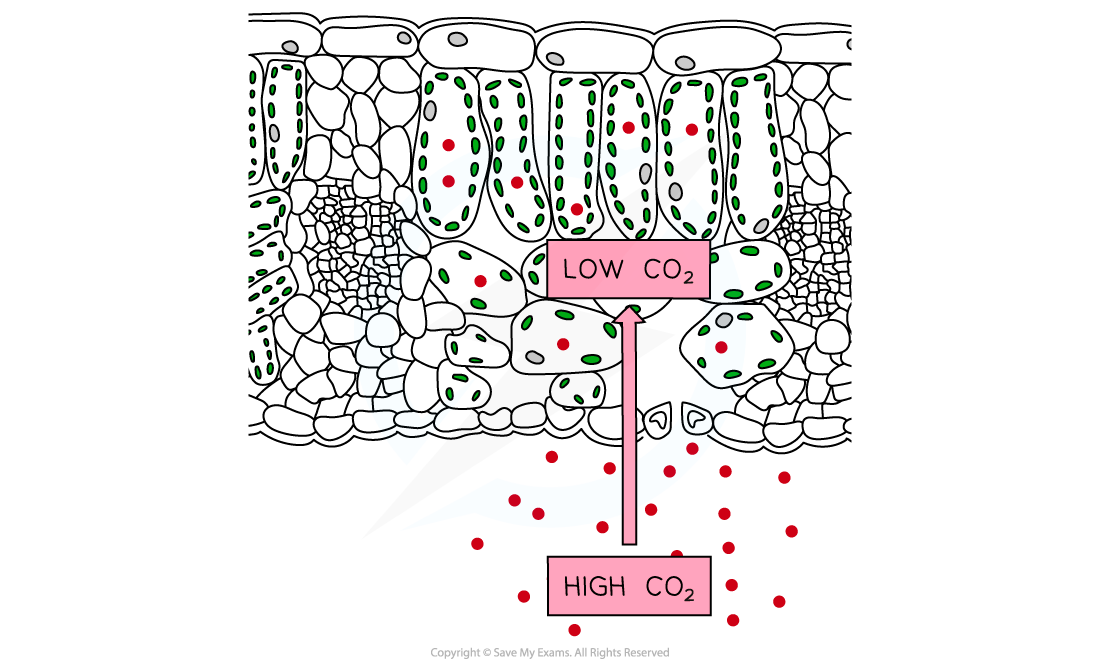
Carbon dioxide diffuses into the leaves of plants down its concentration gradient
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









