- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Physics: HL复习笔记12.1.5 Matter Waves
Matter Waves
- De Broglie proposed that electrons travel through space as a wave
- This would explain why they can exhibit behaviour such as diffraction
- He therefore suggested that electrons must also hold wave properties, such as wavelength
- This came to be known as the de Broglie wavelength
- However, he realised all particles can show wave-like properties, not just electrons
- He hypothesised that all moving particles have a matter wave associated with them
- This is known as the de Broglie wavelength, and can be defined as:
The wavelength associated with a moving particle
- The majority of the time, and for everyday objects travelling at normal speeds, the de Broglie wavelength is far too small for any quantum effects to be observed
- A typical electron in a metal has a de Broglie wavelength of about 10 nm
- Therefore, quantum mechanical effects will only be observable when the width of the sample is around that value
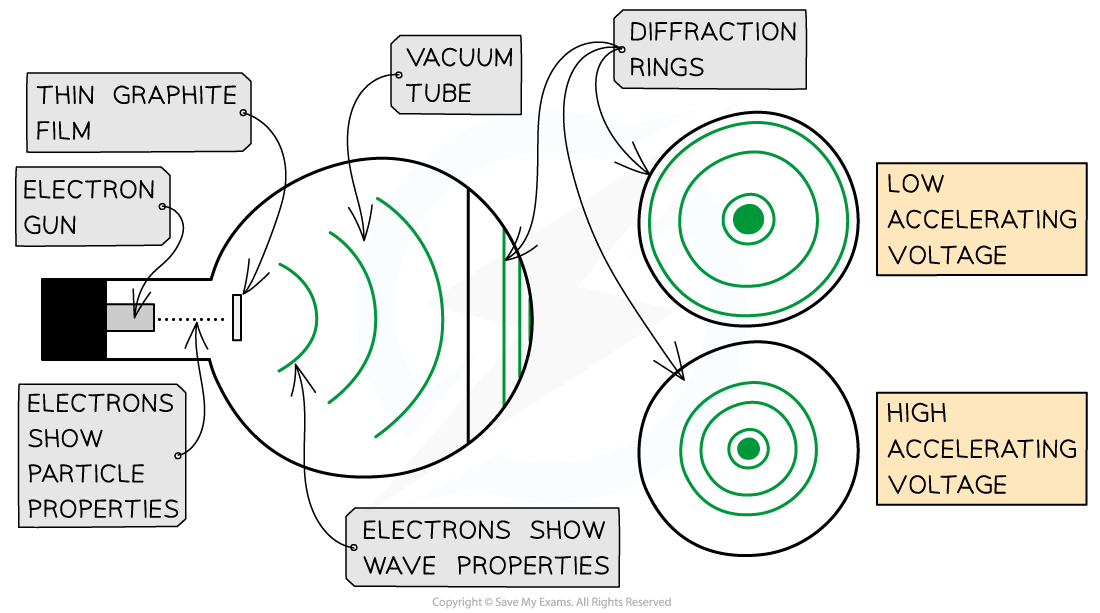
- The electron diffraction tube can be used to investigate how the wavelength of electrons depends on their speed
- The smaller the radius of the rings, the smaller the de Broglie wavelength of the electrons
- As the voltage is increased:
- The energy of the electrons increases
- The radius of the diffraction pattern decreases
- This shows as the speed of the electrons increases, the de Broglie wavelength of the electrons decreases
- Using ideas based upon the quantum theory and Einstein’s theory of relativity, de Broglie suggested that the momentum (p) of a particle and its associated wavelength (λ) are related by the equation:
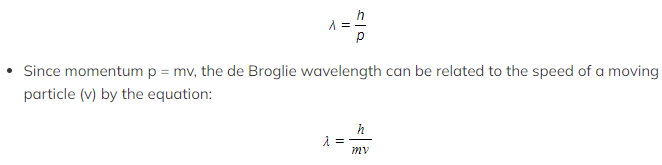 Kinetic Energy
Kinetic Energy
- Since kinetic energy E = ½ mv2
- Momentum and kinetic energy can be related by:
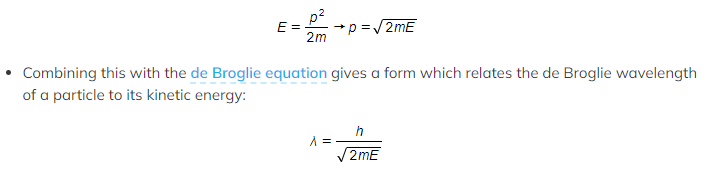
- Where:
- λ = the de Broglie wavelength (m)
- h = Planck’s constant (J s)
- p = momentum of the particle (kg m s-1)
- E = kinetic energy of the particle (J)
- m = mass of the particle (kg)
- v = speed of the particle (m s-1)
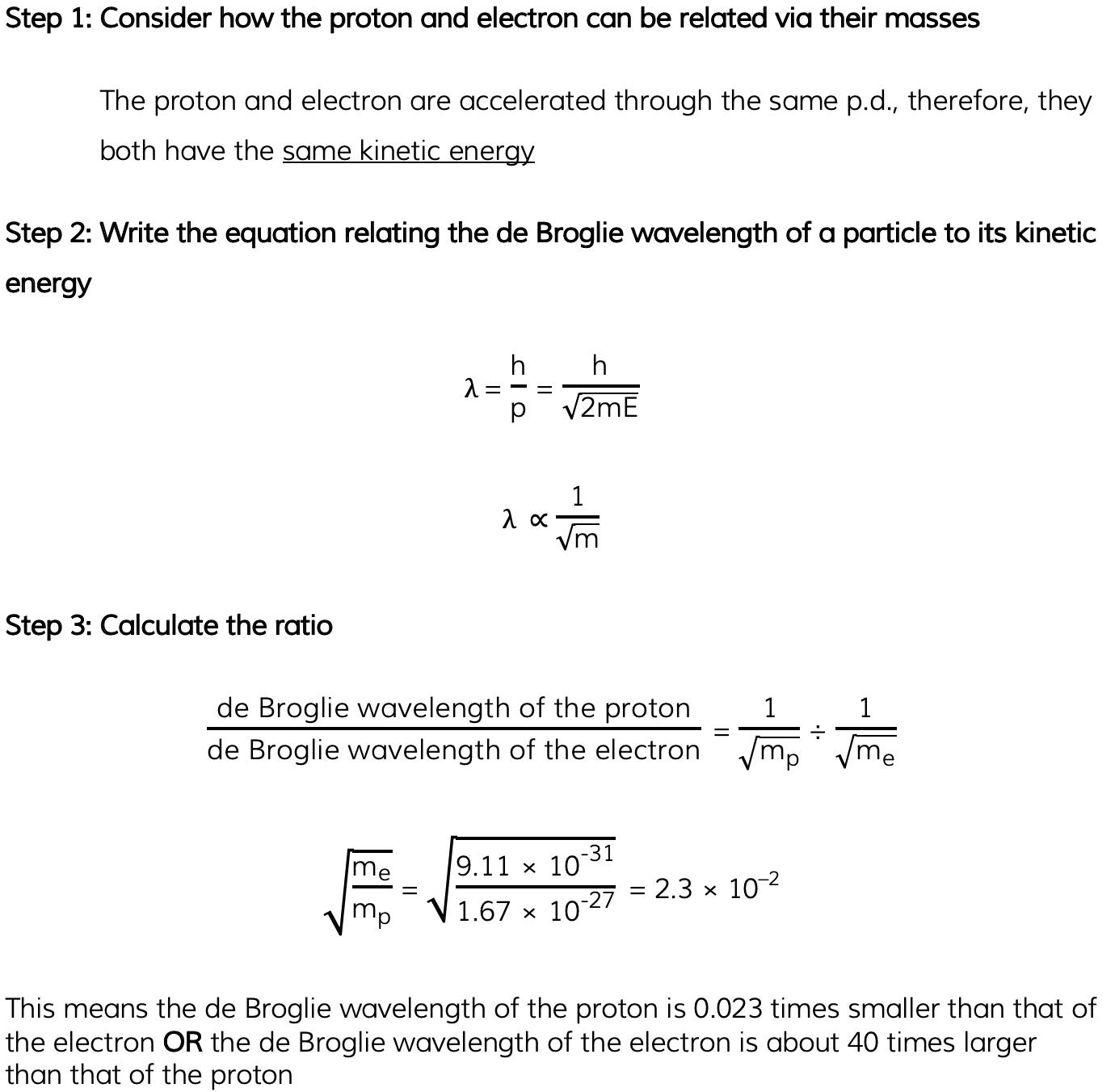
 Mass of a proton = 1.67 × 10–27 kg
Mass of a proton = 1.67 × 10–27 kg
- Mass of an electron = 9.11 × 10–31 kg
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








