- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Physics: HL复习笔记12.1.3 Observing the Photoelectric Effect
Observing the Photoelectric Effect
The photoelectric effect can be observed on a gold leaf electroscope
A plate of metal, usually zinc, is attached to a gold leaf, which initially has a negative charge, causing it to be repelled by a central negatively charged rod
This causes negative charge, or electrons, to build up on the zinc plate
UV light is shone onto the metal plate, leading to the emission of photoelectrons
This causes the extra electrons on the central rod and gold leaf to be removed, so, the gold leaf begins to fall back towards the central rod
This is because they become less negatively charged, and hence repel less
Some notable observations:
Placing the UV light source closer to the metal plate causes the gold leaf to fall more quickly
Using a higher frequency light source does not change the how quickly the gold leaf falls
Using a filament light source causes no change in the gold leaf’s position
Using a positively charged plate also causes no change in the gold leaf’s position
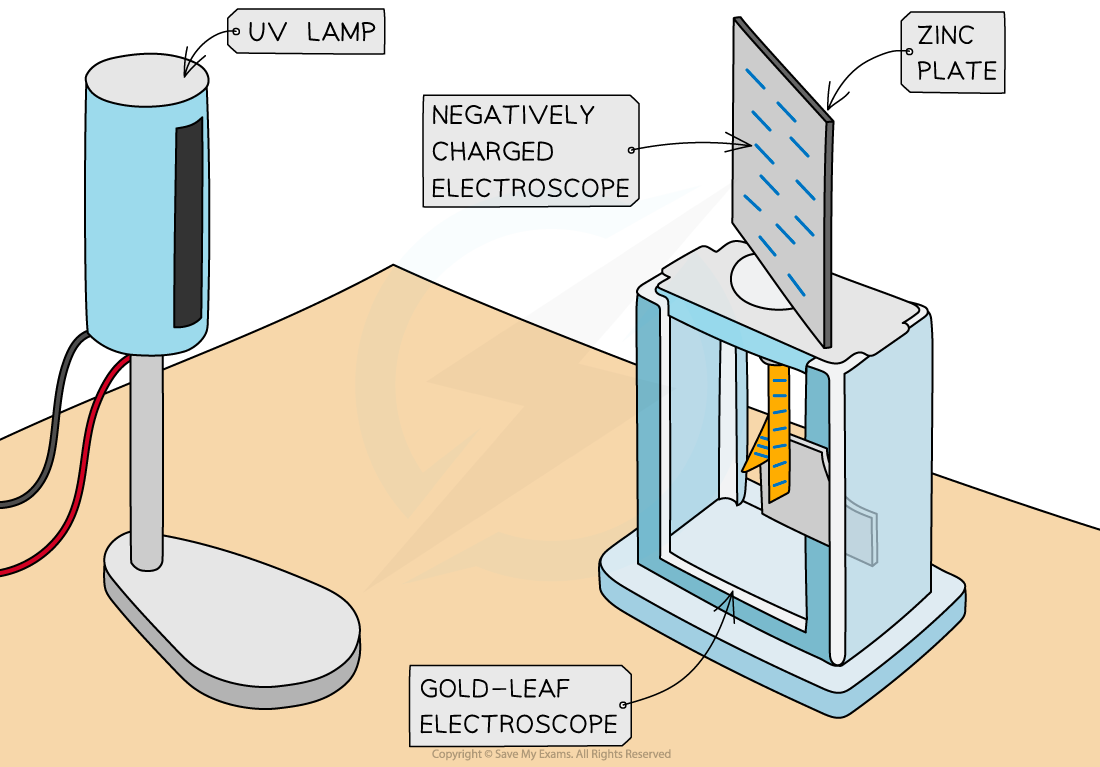
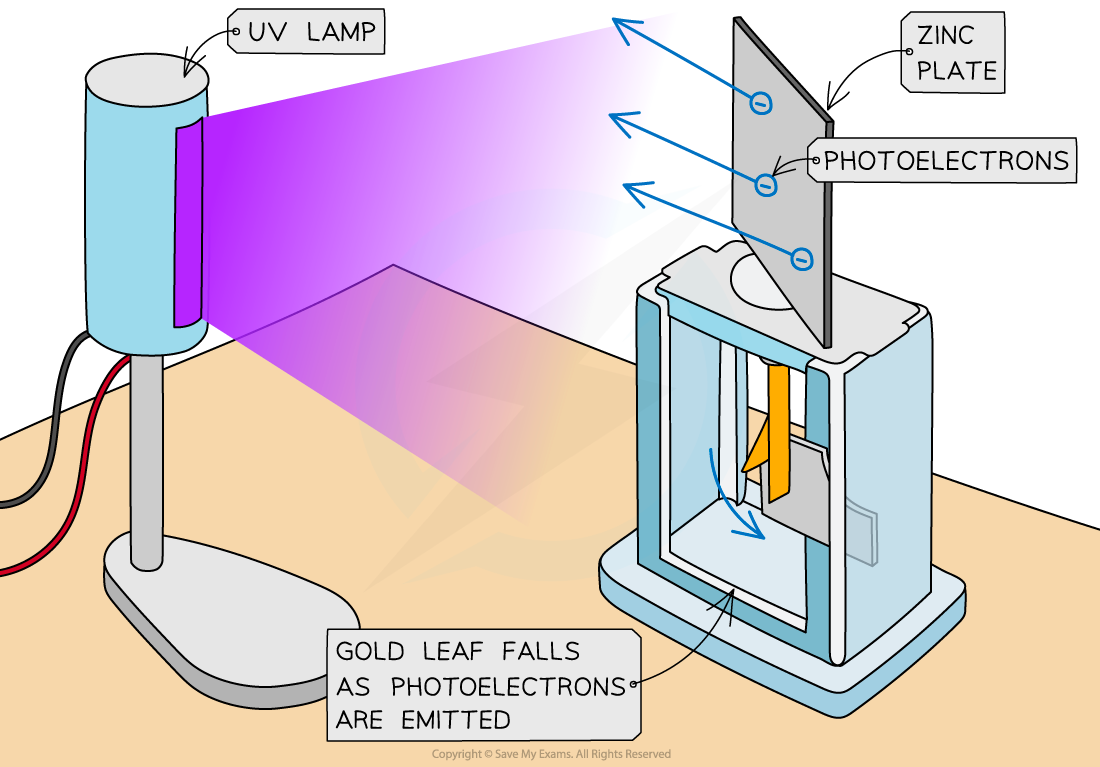
Typical set-up of the gold leaf electroscope experiment
Laws of Photoelectric Emission
The photoelectric effect is evidence that light does not purely behave as a wave
This is demonstrated by the following observations
Observation 1:
Explanation 1:
Placing the UV source closer to the plate increases the intensity incident on the surface of the metal
Increasing the intensity, or brightness, of the incident radiation increases the number of photoelectrons emitted per second
Therefore, the gold leaf loses negative charge more rapidly
Observation 2:
Explanation 2:
The maximum kinetic energy of the emitted electrons increases with the frequency of the incident radiation
In the case of the photoelectric effect, energy and frequency are independent of the intensity of the radiation
So, the intensity of the incident radiation affects how quickly the gold leaf falls, not the frequency
Observation 3:
Explanation 3:
If the incident frequency is below a certain threshold frequency, no electrons are emitted, no matter the intensity of the radiation
A filament light source has a frequency below the threshold frequency of the metal, so, no photoelectrons are released
Observation 4:
Explanation 4:
If the plate is positively charged, that means there is an excess of positive charge on the surface of the metal plate
Electrons are negatively charged, so they will not be emitted unless they are on the surface of the metal
Any electrons emitted will be attracted back by positive charges on the surface of the metal
Observation 5:
Emission of photoelectrons happens as soon as the radiation is incident on the surface of the metal
Explanation 5:
A single photon interacts with a single electron
If the energy of the photon is equal to the work function of the metal, photoelectrons will be released instantaneously
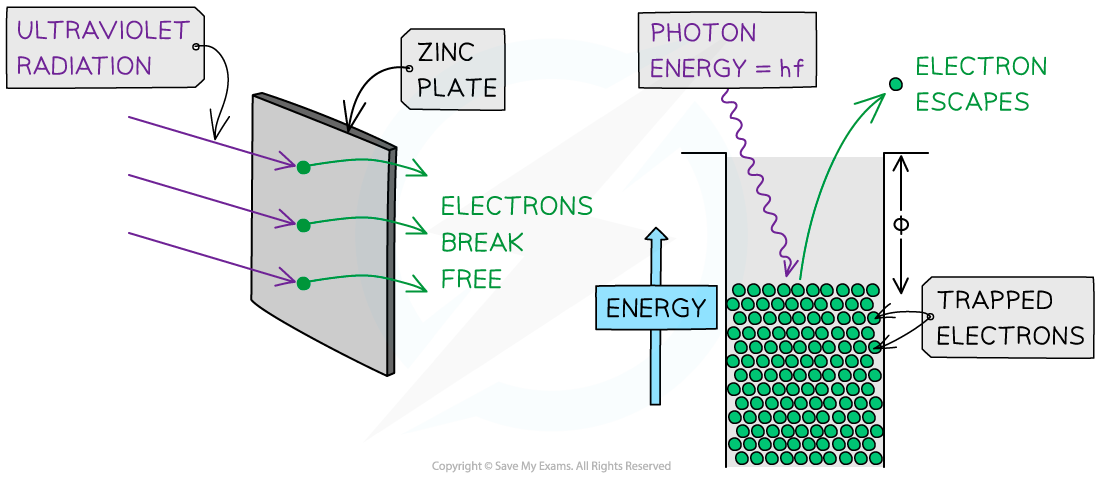
In the photoelectric effect, a single photon may cause a surface electron to be released if it has sufficient energy
Stopping Voltage
Stopping voltage, Vs, is defined as:
The voltage required to stop photoelectron emission from occurring
The photons arriving at the metal plate cause photoelectrons to be emitted
This is called the emitter plate
The electrons that cross the gap are collected at the other metal plate
This is called the collector plate
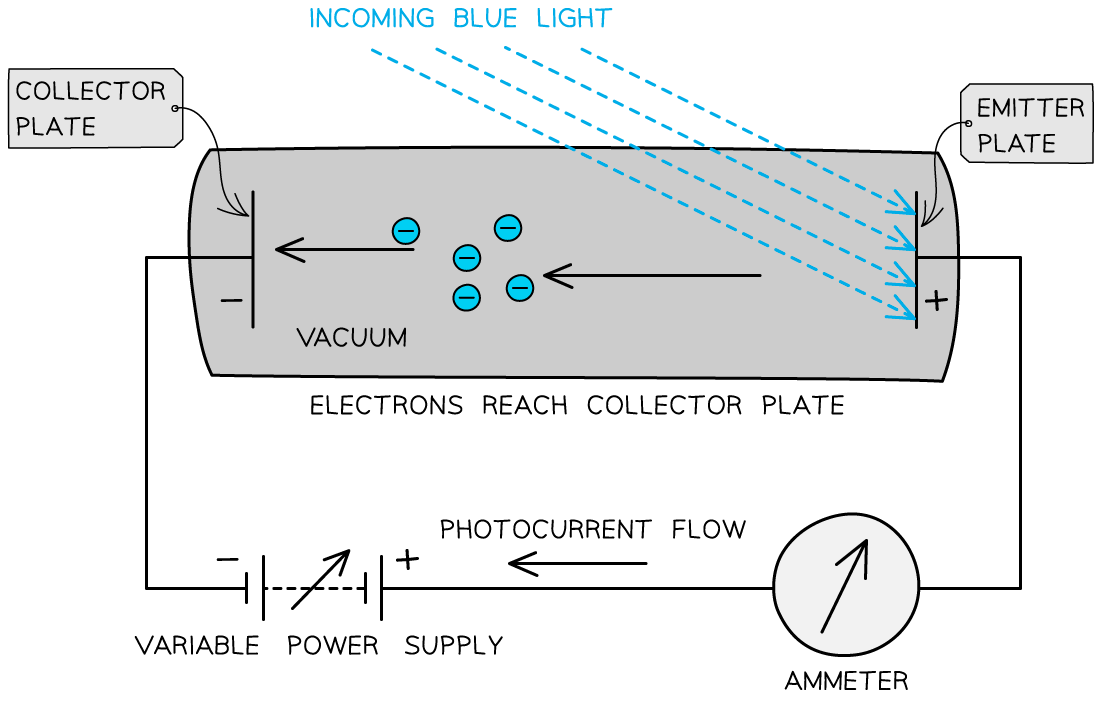
This set-up can be used to determine the maximum kinetic energy of the emitted photoelectrons
The flow of electrons across the gap results in an e.m.f. between the plates that causes a current to flow around the rest of the circuit
Effectively, it becomes a photoelectric cell producing a photoelectric current
If the e.m.f. of the variable power supply is initially zero, the circuit operates only on the photoelectric current
As the supply is turned up, the emitter plate becomes more positive (because it is connected to the positive terminal of the supply)
As a result, electrons leaving the emitter plate are attracted back towards it
This is because the p.d. across the tube opposes the motion of the electrons between the plates
If any electrons escape with enough kinetic energy, they can overcome this attraction and cross to the collector plate
And if they don't have enough energy, they can't cross the gap
By increasing the e.m.f. of the supply, eventually, a p.d. will be reached at which no electrons are able to cross the gap – this is the stopping voltage, Vs
At this point, the energy needed to cross the gap is equal to the maximum kinetic energy KEmax of the electrons
KEmax = eVS
Exam Tip
The observations and explanations of the photoelectric effect are key findings in Physics, which led to a whole new branch of discovery. As such, they are favourites with Examiners. Make sure you have them at your fingertips!
It is important to note that the stopping voltage actually holds a negative value, but since we use it to determine the maximum kinetic energy of the emitted electrons, its sign is not important in calculations, it's acceptable to just quote its magnitude.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









