- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Physics: HL复习笔记7.1.6 Alpha, Beta & Gamma Particles
Alpha, Beta & Gamma Particles
- Some elements have nuclei that are unstable
- This tends to be when the number of nucleons does not balance
- In order to become more stable, they emit particles and/or electromagnetic radiation
- These nuclei are said to be radioactive
- There are three different types of radioactive emission:
- Alpha (α) particles are high energy particles made up of 2 protons and 2 neutrons (the same as a helium nucleus)
- They are usually emitted from nuclei that are too large

Beta-Minus Decay
- Beta (β−) particles are high energy electrons emitted from the nucleus
- β− particles are emitted by nuclei that have too many neutrons
- During beta decay, a neutron changes into a proton and an electron
- The electron is emitted and the proton remains in the nucleus
- A completely new element is formed because the proton number changes
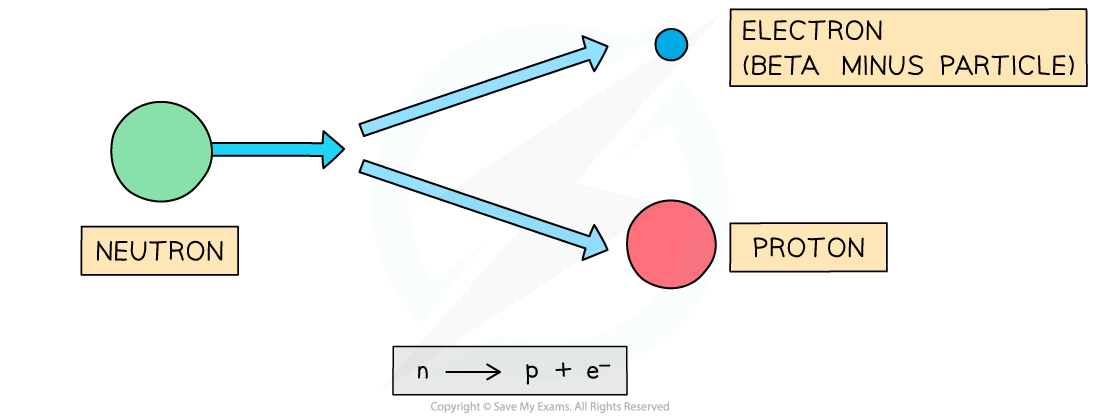
Beta-minus decay often happens in unstable nuclei that have too many neutrons. The nucleon number stays the same, but the proton number increases by one
- An electron has a mass number of 0
- This is because electrons have a negligible mass, compared to neutrons and protons
- Therefore, the nucleon number of the decaying nuclei remains the same
- Electrons have a proton number of -1
- This means that the new nuclei will increase its proton number by 1 in order to maintain the overall proton number before and after the decay
- The following equation shows carbon-14 undergoing beta decay
- It forms nitrogen-14 and a beta minus particle
- Beta minus particles are written as an electron in this equation

The carbon nucleus emits a beta particle, and a neutron changes into a proton. This means its proton number increases by +1 and it changes into a new element, nitrogen
Beta-Plus Decay
- Beta (β+) particles are high energy positrons (anti-matter of electrons) also emitted from the nucleus
- β+ particles are emitted by nuclei that have too many protons
- During beta plus (β+) decay a proton turns into a neutron emitting a positron (anti-electron)
- The positron is emitted and the neutron remains in the nucleus
- A completely new element is formed because the proton number changes
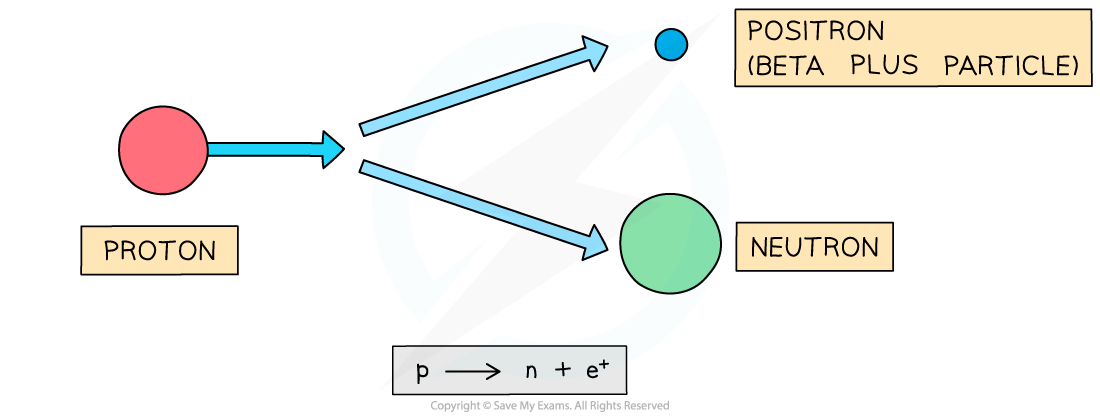
Beta-plus decay often happens in unstable nuclei that have too many protons. The nucleon number stays the same, but the proton number decreases by one
- A positron has a mass number of 0
- This is because the positrons have a negligible mass, just like the electron, compared to neutrons and protons
- Therefore, the nucleon number of the decaying nuclei remains the same
- Positrons have an proton number of +1
- This means that the new nuclei will decrease its proton number by 1 in order to maintain the overall atomic number before and after the decay

- Gamma (γ) rays are high energy electromagnetic waves
- They are emitted by nuclei that need to lose some energy

- If these particles hit other atoms, they can knock out electrons, ionising the atom
- This can cause chemical changes in materials and can damage or kill living cells
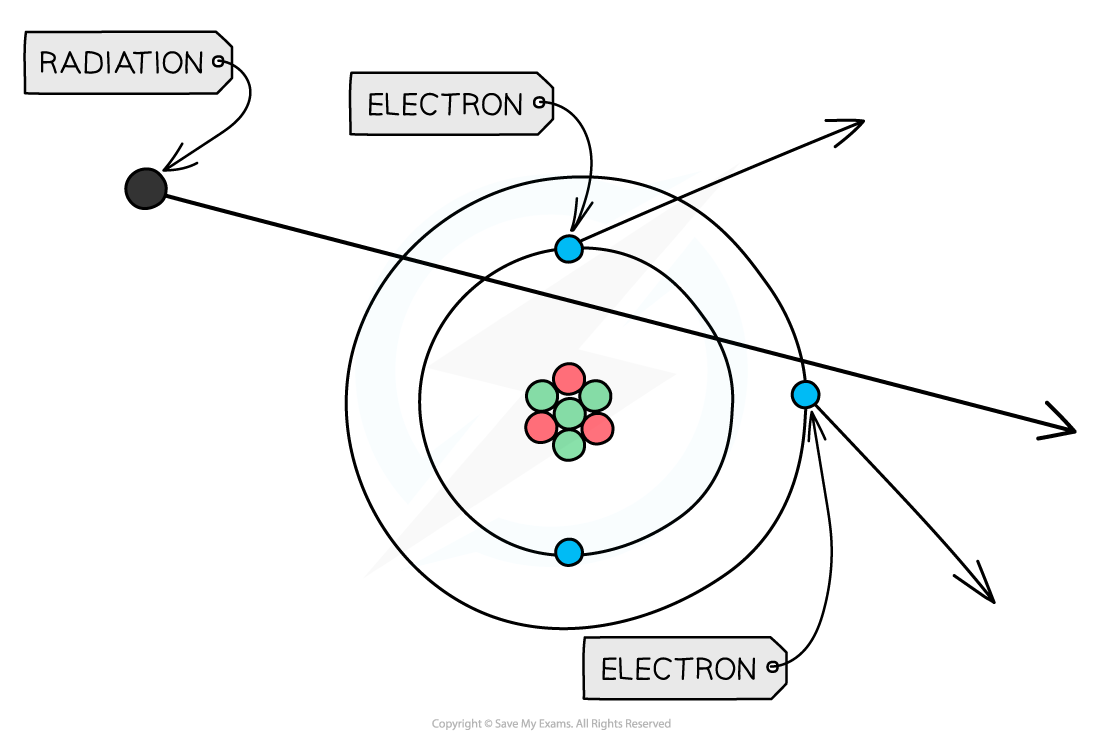
When radiation passes close to atoms, it can knock out electrons, ionising the atom
- The properties of the different types of radiation are summarised in the table below
- Note that charge is often described as 'relative charge'
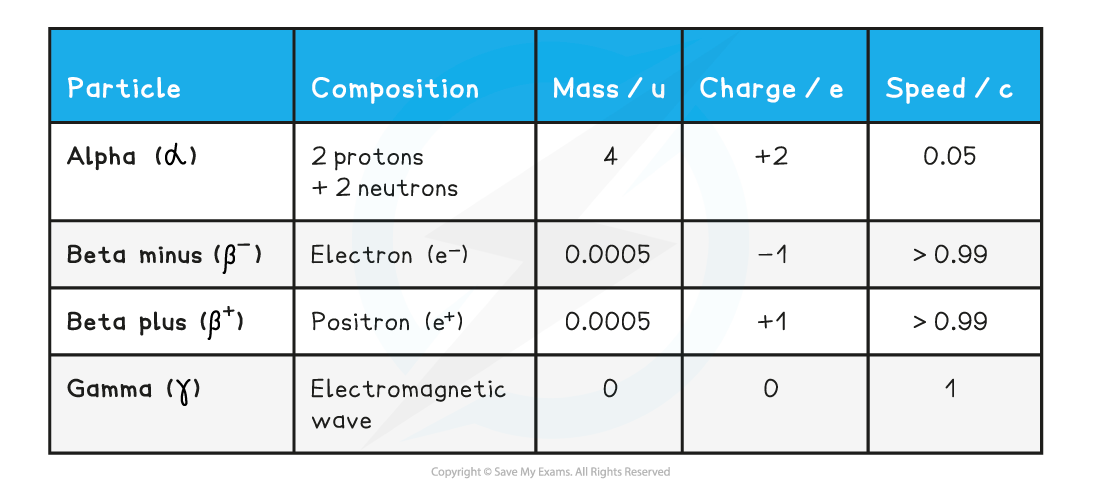
- u is the atomic mass unit (see “Atomic Mass Unit (u)”)
- e is the charge of the electron: 1.60 × 10-19 C
- c is the speed of light: 3 × 108 m s-1
Worked Example
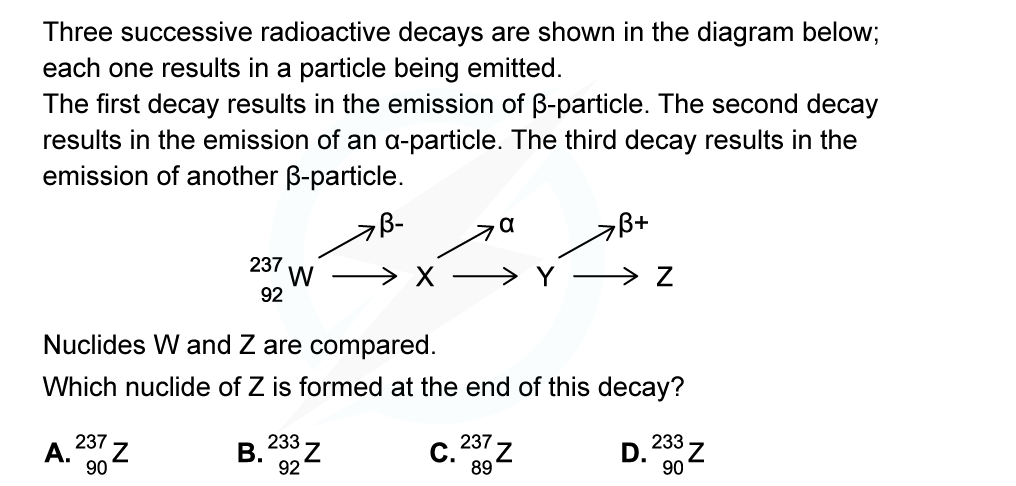
ANSWER: D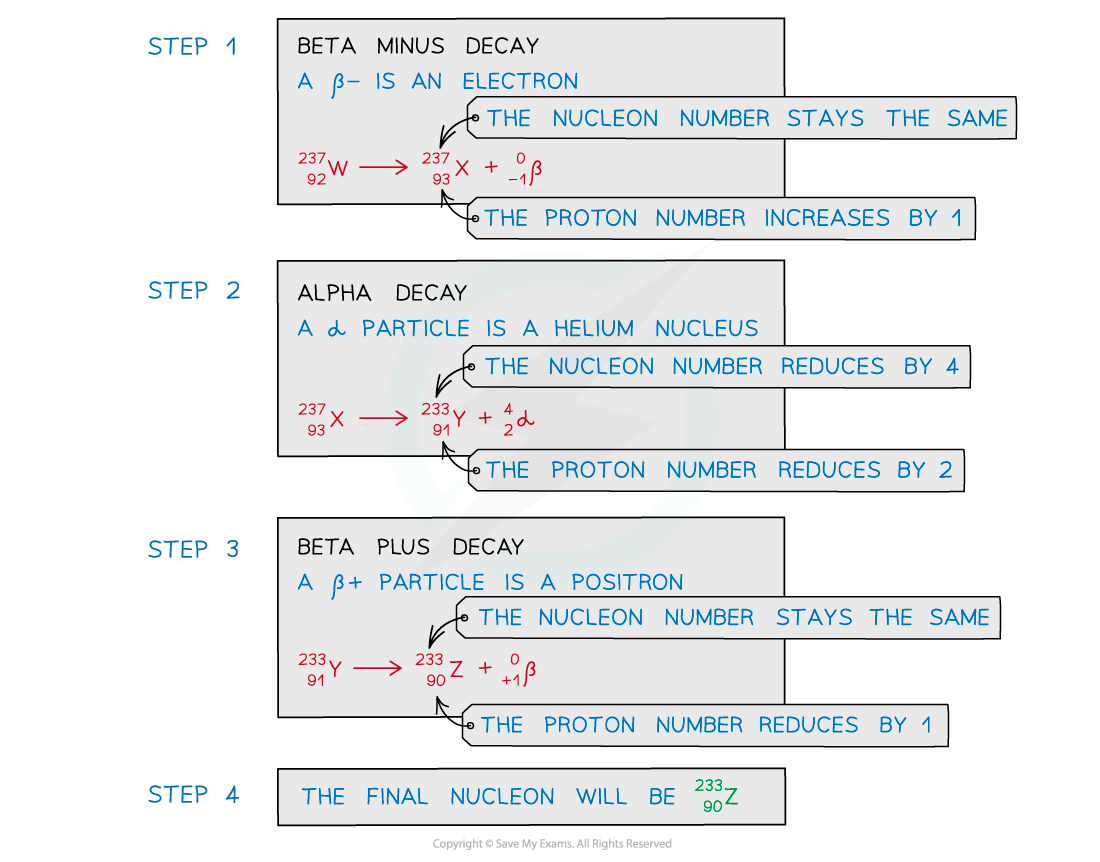
Neutrino Emission
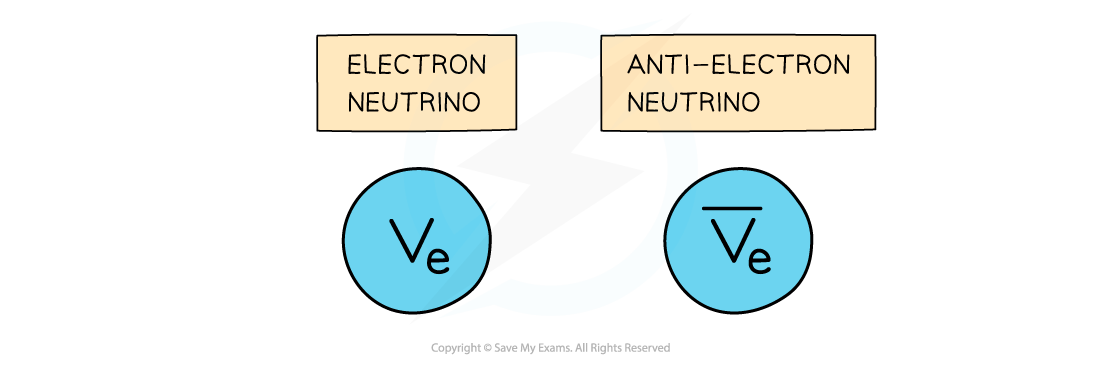
- An electron neutrino is a type of subatomic particle with no charge and negligible mass which is also emitted from the nucleus
- The anti-neutrino is the antiparticle of a neutrino
- Electron anti-neutrinos are produced during β– decay
- Electron neutrinos are produced during β+ decay
Exam Tip
One way to remember which particle decays into which depends on the type of beta emission, think of beta ‘plus’ as the ‘proton’ that turns into the neutron (plus an electron neutrino)
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









