Structure Identification Problems
- The chemists' toolkit includes a range of analytical techniques that enable the structure of compounds to be deduced
Summary table of analytical techniques
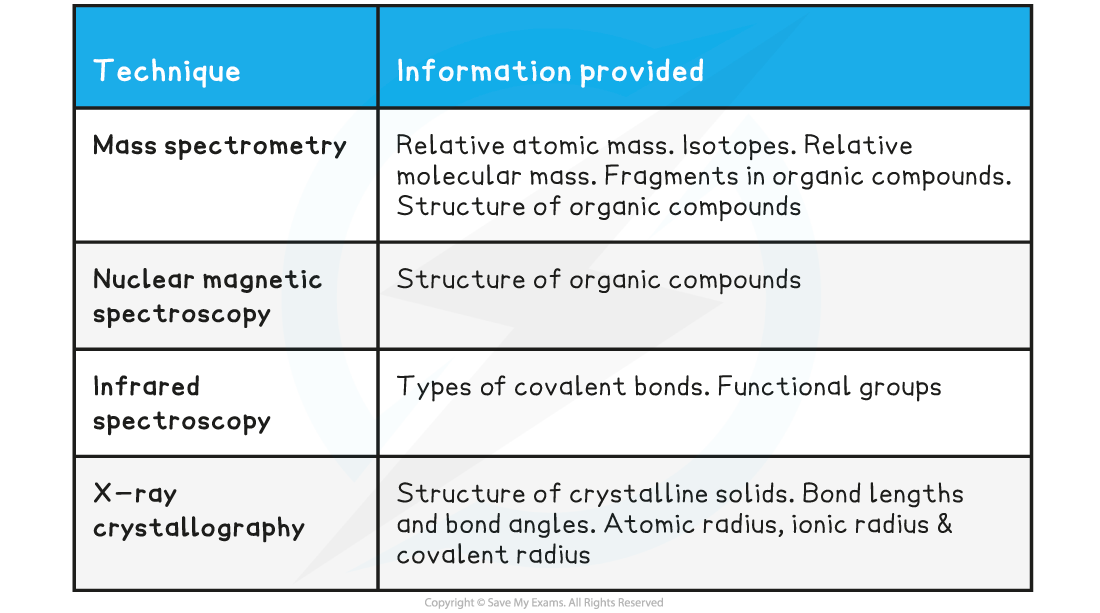
- These techniques are rarely used in isolation, but together provide corroborating evidence for elucidating chemical information on newly discovered or synthetic compounds
- Problem solving typically involves taking multiple pieces of spectroscopic data about the same unknown compound and coming up with a likely structure
Worked Example
An unknown compound, X, of molecular formula,C4H8O, has the following MS, IR and 1H NMR spectra.
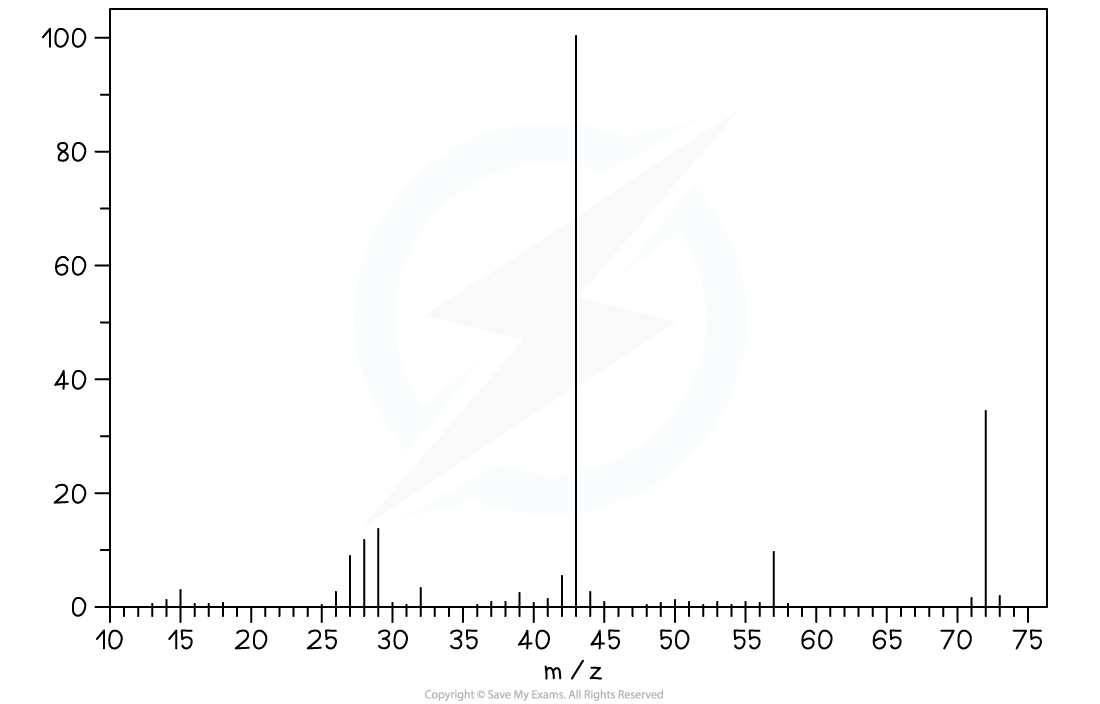
MS of X
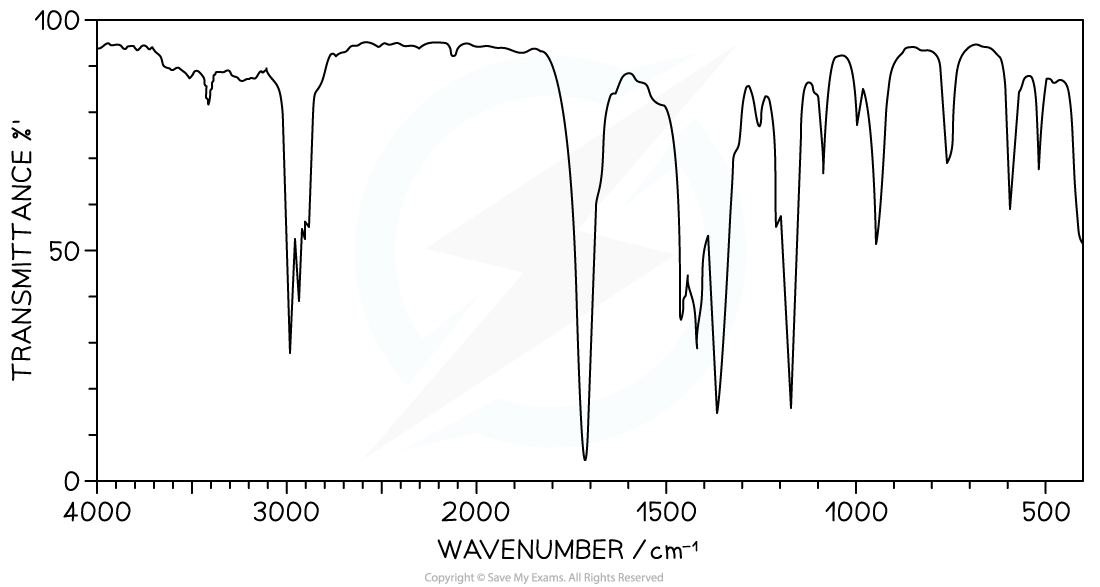
IR spectrum of X
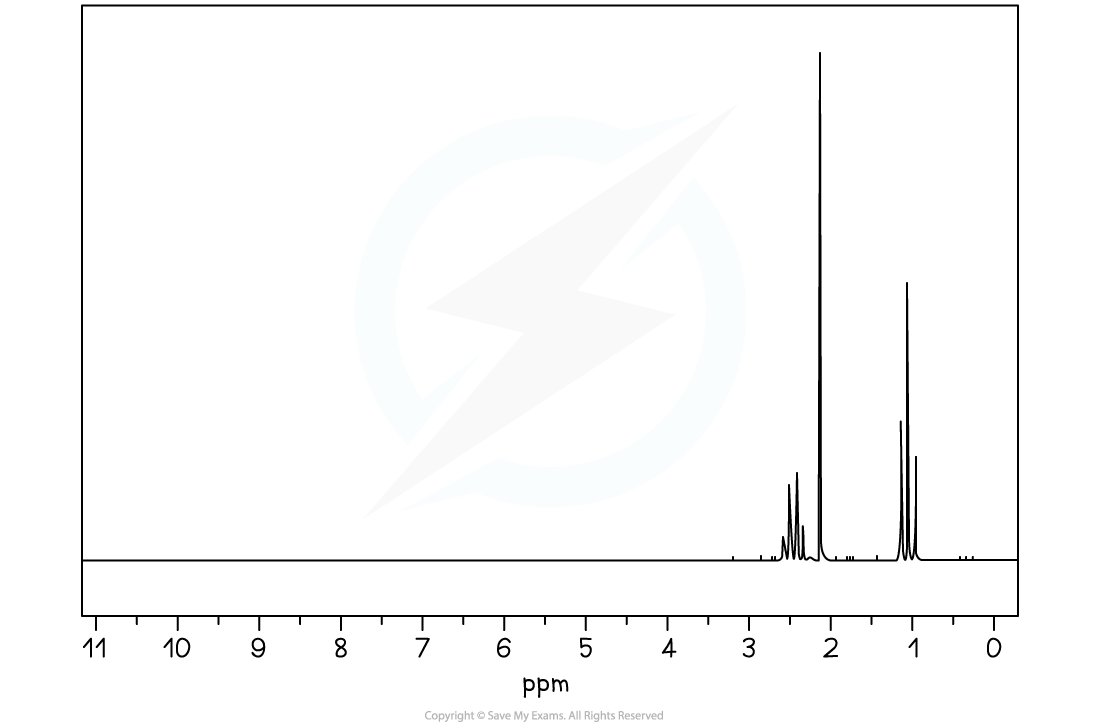
1H NMR spectrum of X
Deduce the structure of X using the information given and any other additional information in the Data booklet. For each spectrum assign as much spectroscopic information as possible.
Answer
Mass Spectrum
-
- The molecular ion peak is at m/z = 72, which corresponds to the relative molecular mass of C4H8O
Mr= (12 × 4) + (8 × 1) +(16) = 72
-
- The large peak at m/z = 43 could correspond to CH3CH2CH2+ or CH3CO+ indicating the loss of CH4O or C2H5 from X, that is (Mr- 43)
- The peak at m/z = 29 could correspond to CH3CH2+ indicating the loss of C2H3O from X, that is (Mr- 29)
IR Spectrum
-
- There is a strong absorption in the range 1700-1750 cm-1 which corresponds to C=O, based on Section 26 of the Data book
- This suggests an aldehyde or ketone is present (it cannot be an ester or carboxylic acid as only one oxygen is in the formula)
1H NMR Spectrum
-
- The 1H NMR spectrum shows three protons environments
- The peak around chemical shift 1.0 ppm could correspond to a proton on the end of a chain, -CH3
- The peaks around chemical shift 2.2 - 2.7 ppm could correspond to a proton next to a carbonyl group RCH2CO-
- The peak splitting is a quartet, singlet and triplet
- A quartet and triplet in the same spectrum usually corresponds to an ethyl group, CH3CH2, following the n+1 rule
- The singlet indicates an isolated proton environment
Putting the information together the structure of X is

The structure of X is butanone









