- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记20.3.2 Cis-Trans & E/Z Isomers
Cis-Trans & E/Z Isomers
- Configurational isomerism can be seen in unsaturated compounds, cyclic structures or compounds that contain at least one asymmetric carbon (sometimes called a chiral centre)
- These structures have the same molecular formula and order of atoms (the atoms are connected similarly to each other) but different shapes
- As previously discussed, these can be grouped into further types of isomers:
- Cis / trans
- E / Z
- Optical
Exam Tip
You may still see the term geometric isomers being used when talking about some configurational isomers This was recommended by IUPAC but it is now obsolete and being replaced with cis-trans isomers and E / Z isomers
Cis / trans isomers
- In saturated compounds, the atoms / functional groups attached to the single, σ-bonded carbons are not fixed in their position due to the free rotation about the C-C σ-bond
- This causes conformational isomers, as previously discussed
- In unsaturated compounds, the groups attached to the C=C carbons remain fixed in their position
- This is because free rotation of the bonds about the C=C bond is not possible due to the presence of a π bond
- Cis / trans nomenclature can be used to distinguish between the isomers
- Cis isomers have two functional groups on the same side of the double bond / carbon ring, i.e. both above the C=C bond or both below the C=C bond
- Trans isomers have two functional groups on opposite sides of the double bond / carbon ring, i.e. one above and one below the C=C bond
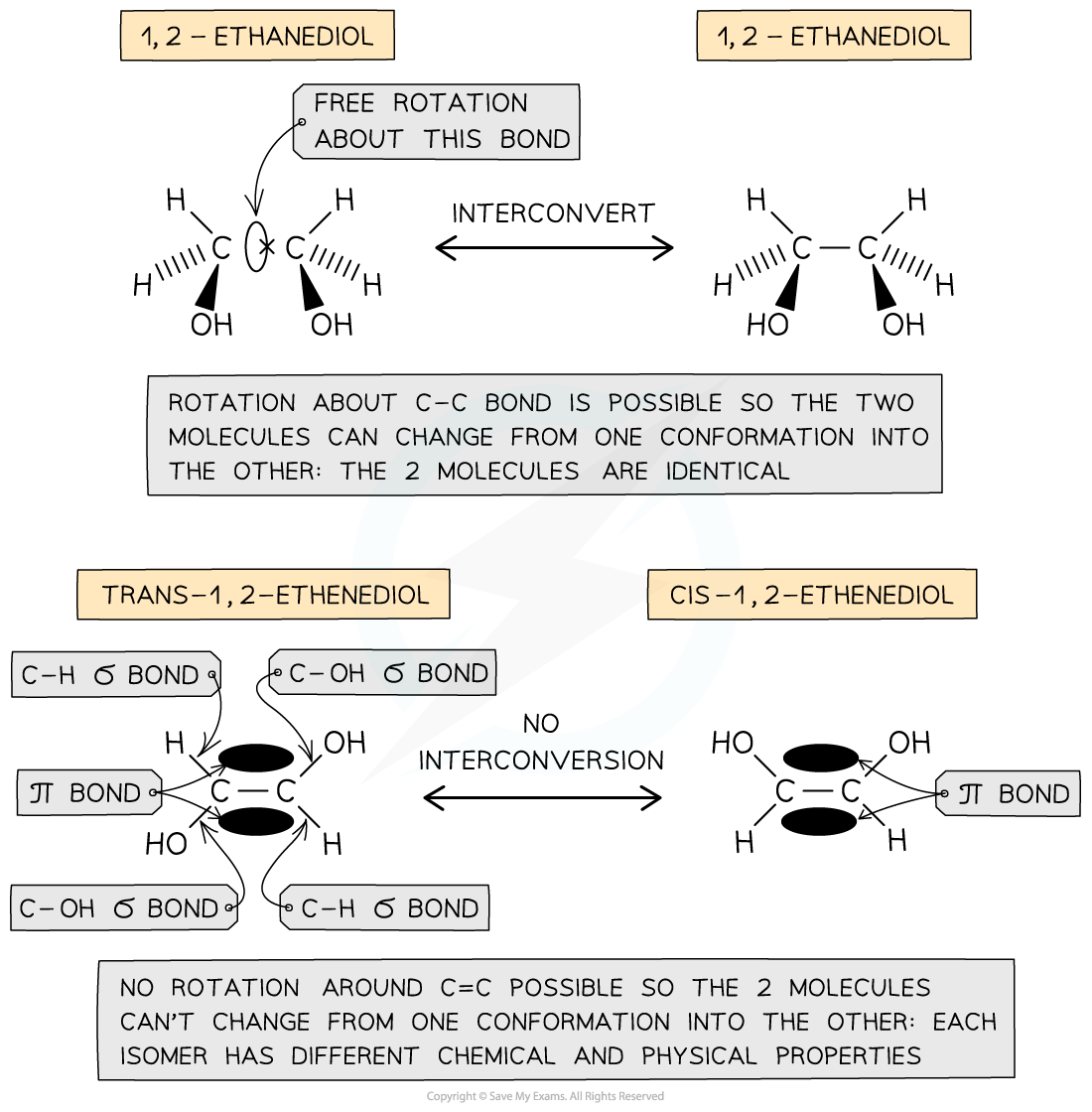
The presence of a π bond in unsaturated compounds restricts rotation about the C=C bond forcing the groups to remain fixed in their position and giving rise to the formation of certain configurational isomers
Naming cis / trans isomers
- For cis / trans isomers to exist, we need two different atoms or groups of atoms on either side of the C=C bond
- This means that 2-methylpropene cannot have cis / trans isomers as the methyl groups are both on the same side of the C=C bond:

2-methylpropene molecules do not have cis / trans isomers
- However, moving one of the methyl groups to the other side of the C=C bond causes cis / trans isomerism:
 But-2-ene does have cis / trans isomers
But-2-ene does have cis / trans isomers
- The atoms or groups of atoms on either side of the C=C bond do not have to be the same for cis / trans isomers:

1-chloroprop-1-ene also shows cis / trans isomerism
- However, the cis / trans naming system starts to fail once we have more than one atom or group of atoms on either side of the C=C bond
- The cis / trans naming system can still be used with three atoms / groups of atoms but only if:
-
- Two of the three atoms or groups of atoms are the same
- These two atoms or groups of atoms are on opposite sides of the double bond
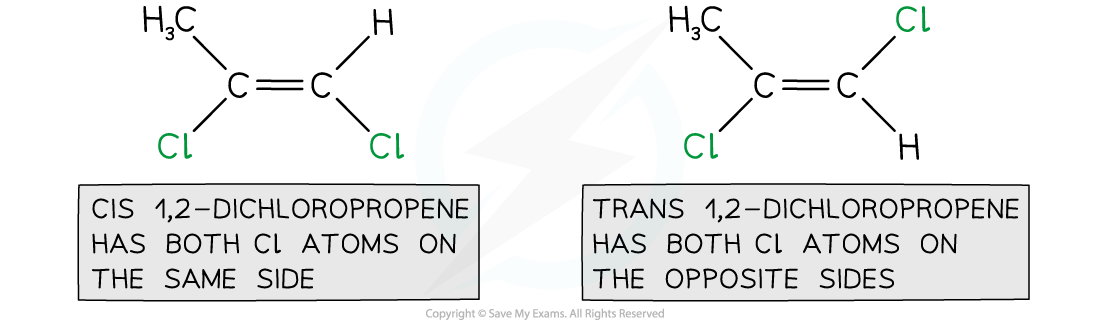
-
- The cis / trans naming system can still be used with three atoms / groups of atoms but only if:
1,2-dichloropropene can be named using cis / trans
-
- The cis / trans naming system cannot be used with three atoms / groups of atoms when they are all different
- This requires the use of the E / Z naming system

- This requires the use of the E / Z naming system
- The cis / trans naming system cannot be used with three atoms / groups of atoms when they are all different
1-bromo-2-chloropropene cannot be named using cis / trans
Exam Tip
Although not part of this topic, the relationship between cis / trans isomers, their packing and melting points is applicable to the Option B: Biochemistry topicCis / trans isomerism affects the intermolecular forces by introducing a dipole moment between molecules, not just London dispersion forces. This will affect the packing of the molecules as well as physical properties such as melting and boiling point
Cyclic cis / trans isomers
- Cis / trans isomerism can also occur in cyclic structures
- Even though cyclic alkanes contain single carbon-carbon bonds, the rigid structure of the ring system does not allow for free rotation
-
- Therefore, cis isomers can occur when the atoms (or groups of atoms) are on the same side of the ring, i.e. both above or both below
- While trans isomers can occur when the atoms (or groups of atoms) are on the opposite side of the ring, i.e. one above and one below
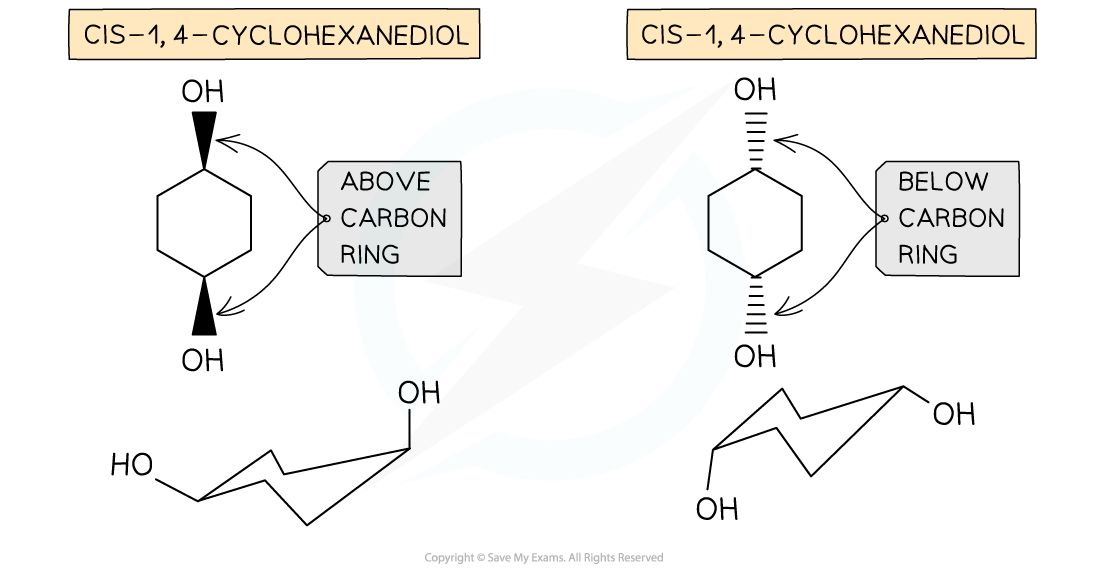
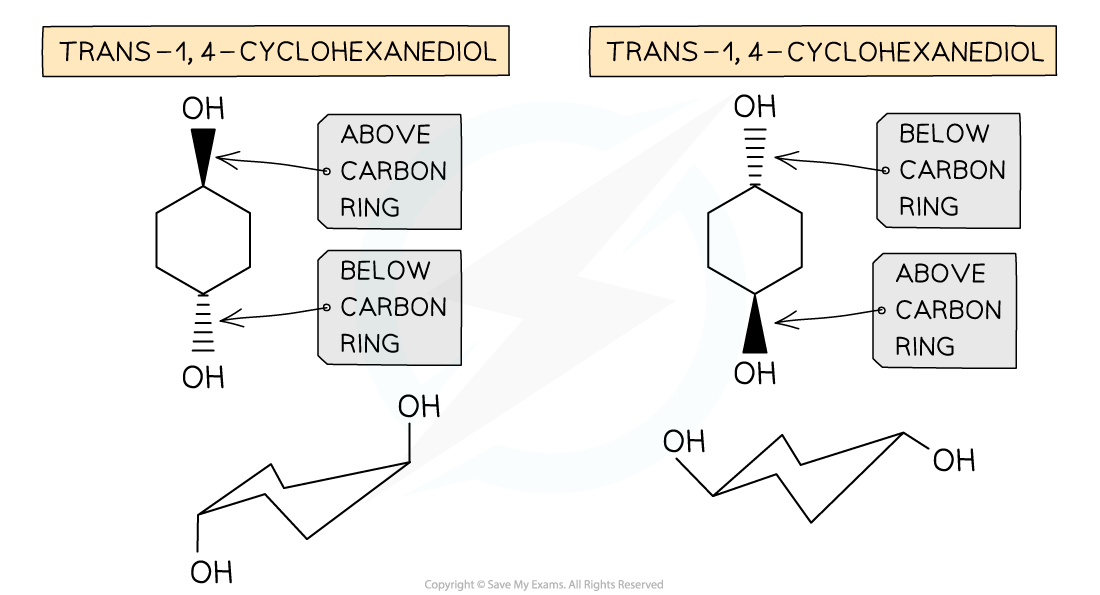
-
- Even though cyclic alkanes contain single carbon-carbon bonds, the rigid structure of the ring system does not allow for free rotation
Cis / trans isomerism in cyclic compounds
E / Z isomers
- To discuss E / Z isomers, we will use an alkene of the general formula C2R4:

The general alkene, C2R4
- When the groups R1, R2, R3 and R4 are all different (i.e. R1 ≠ R2 ≠ R3 ≠ R4), we have to use the E / Z naming system
- This is based on Cahn-Ingold-Prelog (CIP) priority rules
- To do this, we look at the atomic number of the first atom attached to the carbon in question
- The higher the atomic number; the higher the priority
- For example, 2-bromo-1-propen-1-ol has four different atoms or groups of atoms attached to the C=C bond
- This means that it can have two different displayed formulae:
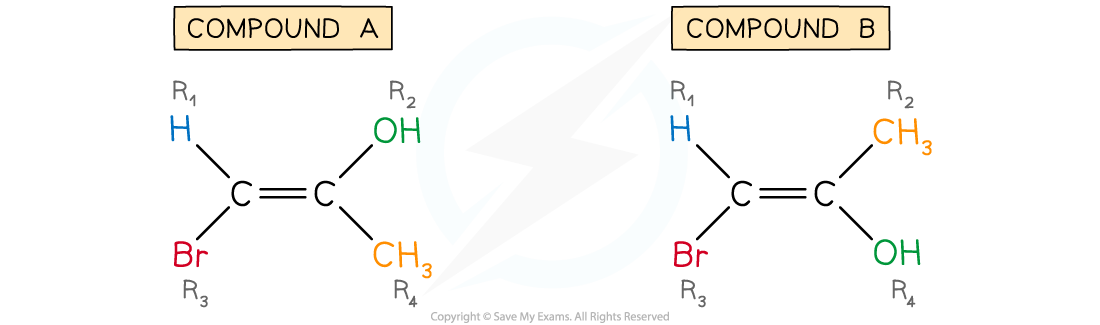
2-Bromo-1-propen-1-ol (compounds A and B)
Compound A
- Step 1: Apply the CIP priority rules
- Look at R1 and R3:
- Bromine has a higher atomic number than hydrogen so bromine has priority
- Look at R2 and R4:
- Oxygen has a higher atomic number than carbon so oxygen has priority
- Look at R1 and R3:
- Step 2: Deduce E or Z
- E isomers have the highest priority groups on opposite sides of the C=C bond, i.e. one above and one below
- The E comes from the German word "entgegen" meaning opposite
- Z isomers have the highest priority groups on the same side of the C=C bond, i.e. both above or both below
- The Z comes from the German word "zusammen" meaning together
- In compound A, the two highest priority groups are on opposite sides (above and below) the C=C bond
- Therefore, compound A is E-2-bromo-1-propen-1-ol
- E isomers have the highest priority groups on opposite sides of the C=C bond, i.e. one above and one below
Compound B
- Step 1: Apply the CIP priority rules
- Look at R1 and R3:
- Bromine has a higher atomic number than hydrogen so bromine has priority
- Look at R2 and R4:
- Oxygen has a higher atomic number than carbon so oxygen has priority
- Look at R1 and R3:
- Step 2: Deduce E or Z
- In compound B, the two highest priority groups are on the same side (both below) the C=C bond
- Therefore, compound B is Z-2-bromo-1-propen-1-ol
- In compound B, the two highest priority groups are on the same side (both below) the C=C bond
More complicated E / Z isomers
- Compound X exhibits E / Z isomerism:
 Compound X
Compound X
- Step 1: Apply the CIP priority rules
- Look at R1 and R3:
- Carbon is the first atom attached to the C=C bond, on the left hand side
- Look at R2 and R4:
- Carbon is the first atom attached to the C=C bond, on the right hand side
- This means that we cannot deduce if compound X is an E or Z isomer by applying the CIP priority rules to the first atom attached to the C=C bond
- Therefore, we now have to look at the second atoms attached
- Look again at R1 and R3:
- The second atoms attached to R1 are hydrogens and another carbon
- The second atoms attached to R3 are hydrogens and bromine
- We can ignore the hydrogens as both R groups have hydrogens
- Bromine has a higher atomic number than carbon, so bromine is the higher priority
- Therefore, the CH2Br group has priority over the CH3CH2 group
- Look again at R2 and R4:
- The second atoms attached to R2 are hydrogens
- The second atoms attached to R3 are hydrogens and an oxygen
- Oxygen has a higher atomic number than hydrogen, so oxygen is the higher priority
- Therefore, the CH2OH group has priority over the CH3 group
- Look at R1 and R3:
- Step 2: Deduce E or Z
- In compound X, the two highest priority groups are on the same side (both below) the C=C bond
- Therefore, compound X is the Z isomer
- In compound X, the two highest priority groups are on the same side (both below) the C=C bond
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









