- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记20.1.2 Electrophilic Addition Reactions
Electrophilic Addition Mechanism
Electrophilic Addition
- Electrophilic addition is the addition of an electrophile (or Lewis acid) to an alkene double bond, C=C
- The alkene double bond, C=C, is an area of high electron density which makes it susceptible to attack by electrophiles
- The C=C bond breaks forming a single C-C bond and 2 new bonds from each of the two carbon atoms
- Electrophilic addition reactions include the addition of:
- Hydrogen, H2 (g)
- Steam, H2O (g)
- Hydrogen halides, HX
- Halogens, X2
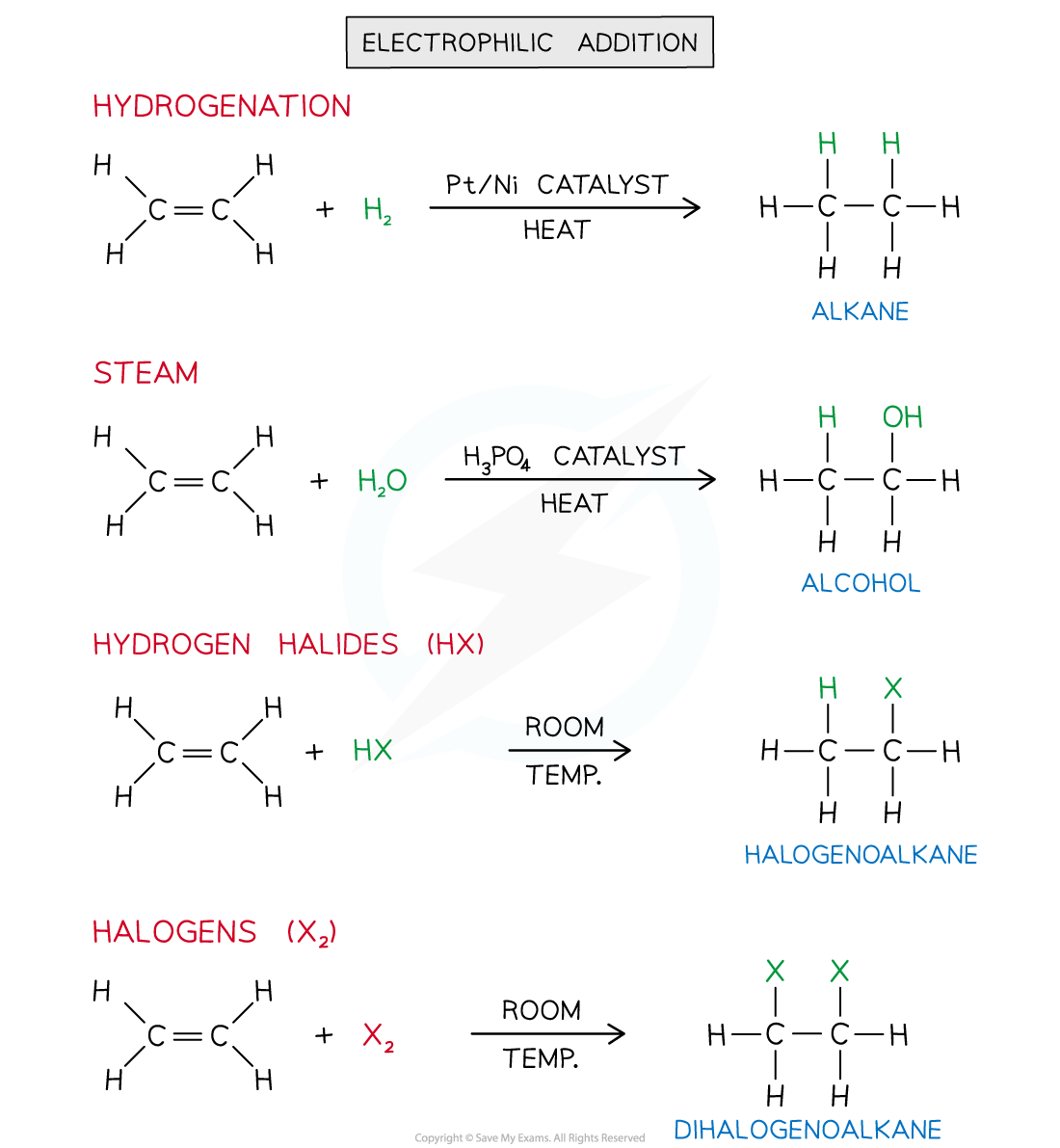
Alkene electrophilic addition reaction overview
Electrophilic addition of hydrogen halides
- A hydrogen halide molecule is polar as the hydrogen and halogen atoms have different electronegativities
- For example, in a molecule of hydrogen bromide, HBr, the bromine atom has a stronger pull on the electrons in the H-Br bond
- As a result of this, the Br atom has a partial negative and the H atom a partial positive charge
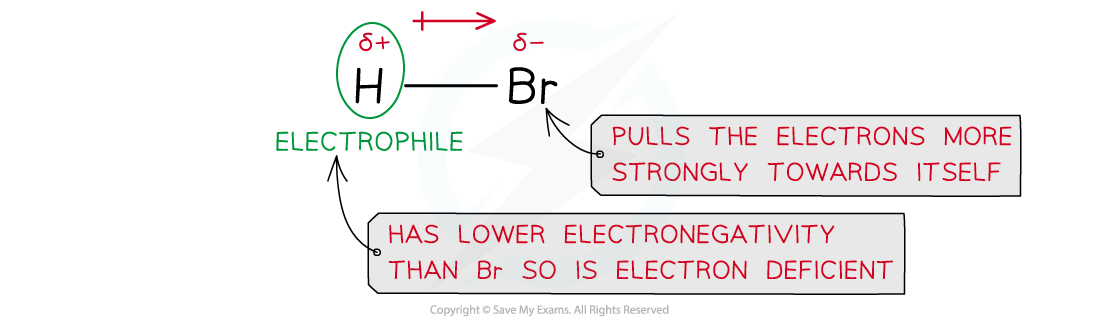
Due to differences in electronegativities of the hydrogen and bromine atom, HBr is a polar molecule
- In electrophilic addition reactions with hydrogen halides, the H atom acts as an electrophile and Lewis acid by accepting a pair of electrons from the C=C bond in the alkene
- The H-Br bond breaks heterolytically, forming a Br- ion
- This results in the formation of a highly reactive carbocation intermediate which reacts with the bromide ion, Br-
- For example, the mechanism for the electrophilic addition of hydrogen bromide and ethene is:
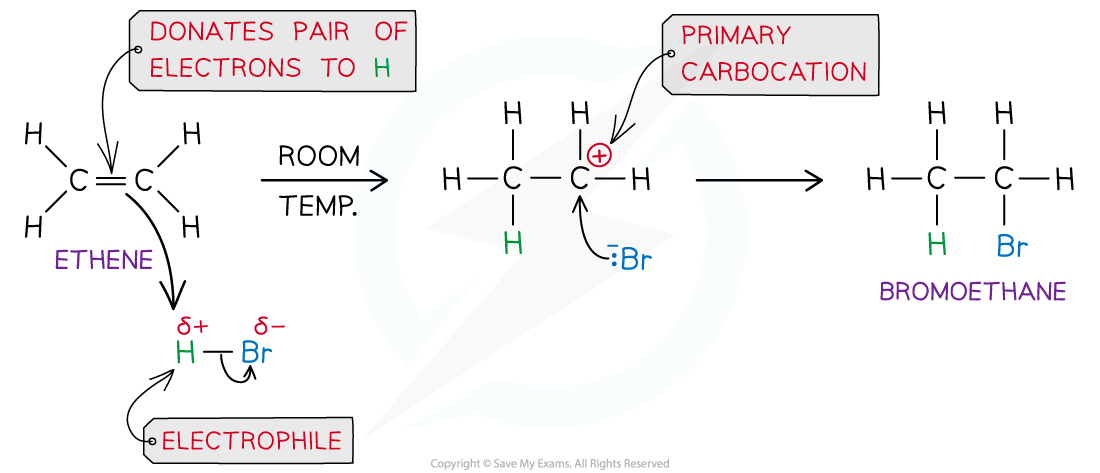
Electrophilic addition reaction of HBr and ethene to form bromoethane
Exam Tip
For electrophilic addition mechanisms, the curly arrows must:
- Be double-headed to show the movement of a pair of electrons
- Start from a lone pair of electrons or an area of high electron density, e.g. the C=C bond
- Move towards a δ+ electrophile or the positive charge of a carbocation
Examiners often comment about the poor and incorrect use of curly arrows in organic mechanisms
Electrophilic addition of halogens
- The mechanism for the electrophilic addition of halogens (and hydrogen) is the same as the electrophilic addition of hydrogen halides with one key exception:
- Hydrogen halide molecules have a permanent dipole (as shown above)
- Halogen molecules have a temporary (or induced) dipole caused by the repulsion of the halogens electrons by the high electron density C=C bond
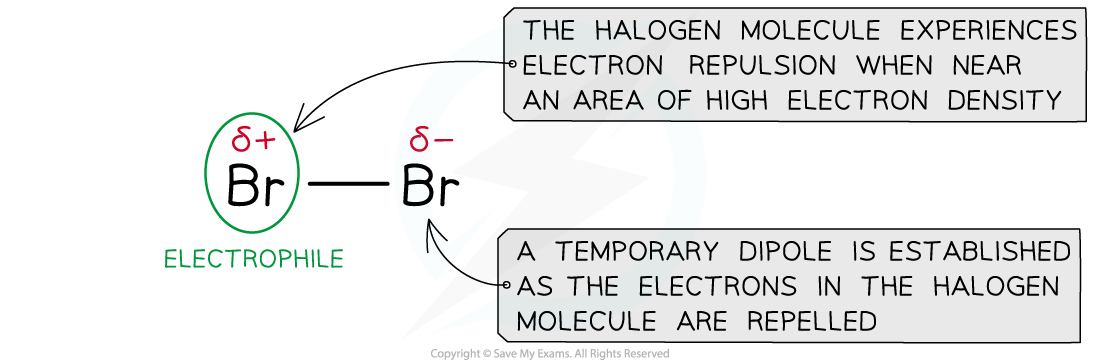
The temporary (or induced) dipole in a halogen molecule
Electrophilic addition of interhalogens
- Interhalogens are compounds that contain two or more different type of halogens
- The mechanism for the electrophilic addition of interhalogens is the same as the electrophilic addition of hydrogen halides
- Just like hydrogen halide molecules, interhalogens have a permanent dipole
- Differences between the electronegativity of the halogens determine which halogen will become the δ+ electrophile
- The electronegativity increases as you move up the halogens, F > Cl > Br > I

The polarity of interhalogen molecules
Exam Tip
The electrophilic addition reactions of alkenes with hydrogen halides, halogens and interhalogens are the same. The difference is whether the electrophile is due to a permanent or temporary dipole
Markovnikov’s Rule
- Carbocations are positively charged carbon atoms with only three covalent bonds instead of four
- There are three types of carbocations: primary, secondary and tertiary
Inductive effect
- The alkyl groups attached to the positively charged carbon atoms are ‘electron donating groups’
- This is also known as the inductive effect of alkyl groups
- The inductive effect is illustrated by the use of arrowheads on the bonds to show the alkyl groups pushing electrons towards the positively charged carbon
- This causes the carbocation to become less positively charged
- As a result of this, the charge is spread around the carbocation which makes it energetically more stable
- This means that tertiary carbocations are the most stable as they have three electron-donating alkyl groups which energetically stabilise the carbocation
- Due to the positive charge on the carbon atom, carbocations are electrophiles
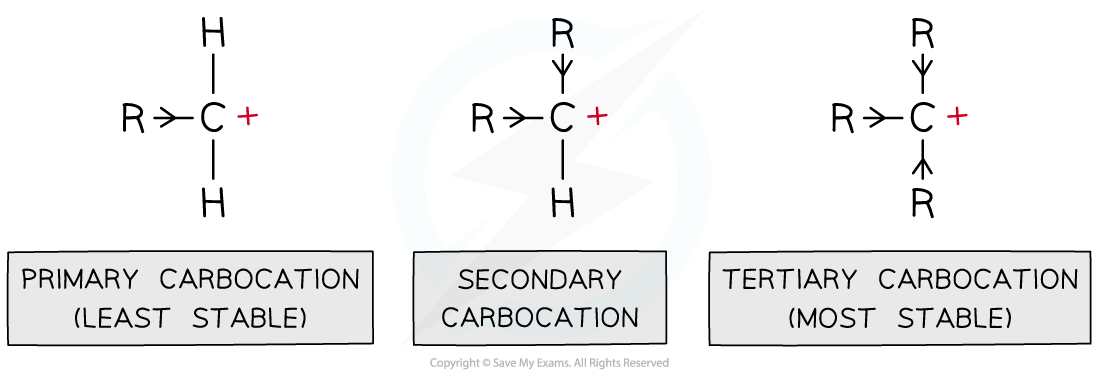
Alkyl groups push electron density towards the carbocation making it energetically more stable; the more alkyl groups the carbocation is bonded to, the more stabilised it is
Markovnikov’s rule
- Markovnikov’s rule predicts the outcome of electrophilic addition reactions and states that:
- In an electrophilic addition reaction of a hydrogen halide (HX) to an alkene, the halogen ends up bonded to the most substituted carbon atom
- In an electrophilic addition reaction of an interhalogen to an alkene, the most electronegative halogen ends up bonded to the most substituted carbon atom
- Markovnikov addition applies to electrophilic addition reactions with unsymmetrical alkanes, e.g. propene and but-1-ene
- Markovnikov addition favours the formation of the major product
- Anti-Markovnikov addition favours the formation of the minor product
- In electrophilic addition reactions, an electrophile reacts with the double bond of alkenes (as previously discussed)
- The mechanism for electrophilic addition reactions with unsymmetrical alkenes is slightly different, e.g. propene + hydrogen bromide
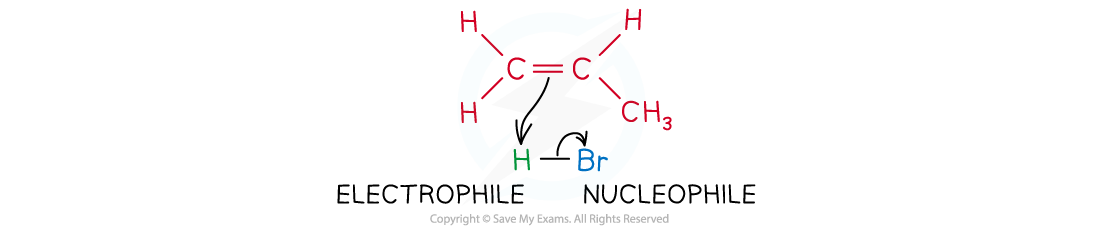
The electrophile reacts with the electron-rich C-C double bond
- The electrophile can attach in two possible ways:
- Breaking the C=C bond and attaching to the the least substituted carbon
- This will give the most stable carbocation as an intermediate that will form the major product
- Breaking the C=C bond and attaching to the the most substituted carbon
- This will give the least stable carbocation as an intermediate that will form the minor product
- Breaking the C=C bond and attaching to the the least substituted carbon

The major and minor carbocation intermediates formed during the reaction of propene and hydrogen bromide
- The nucleophile will bond to the positive carbon atom of the carbocation
- The more stable carbocation produces the major product
- The less stable carbocation produces the minor product
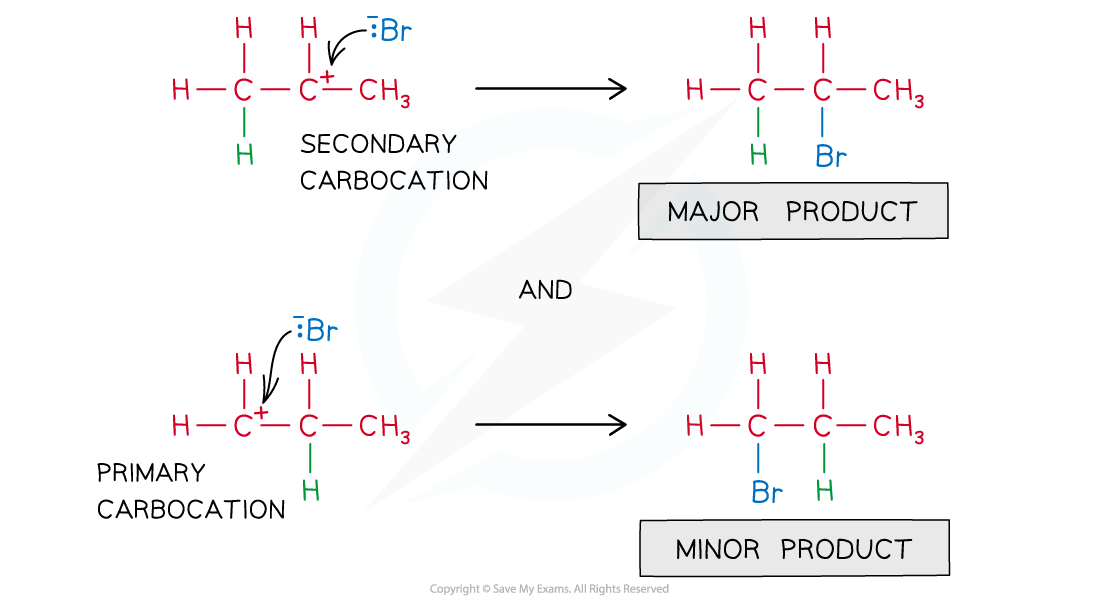
Formation of the major and minor products of the reaction of propene with hydrogen bromide
- The mechanism for the electrophilic addition of hydrogen bromide to propene, showing the formation of the major and minor products can be shown as:
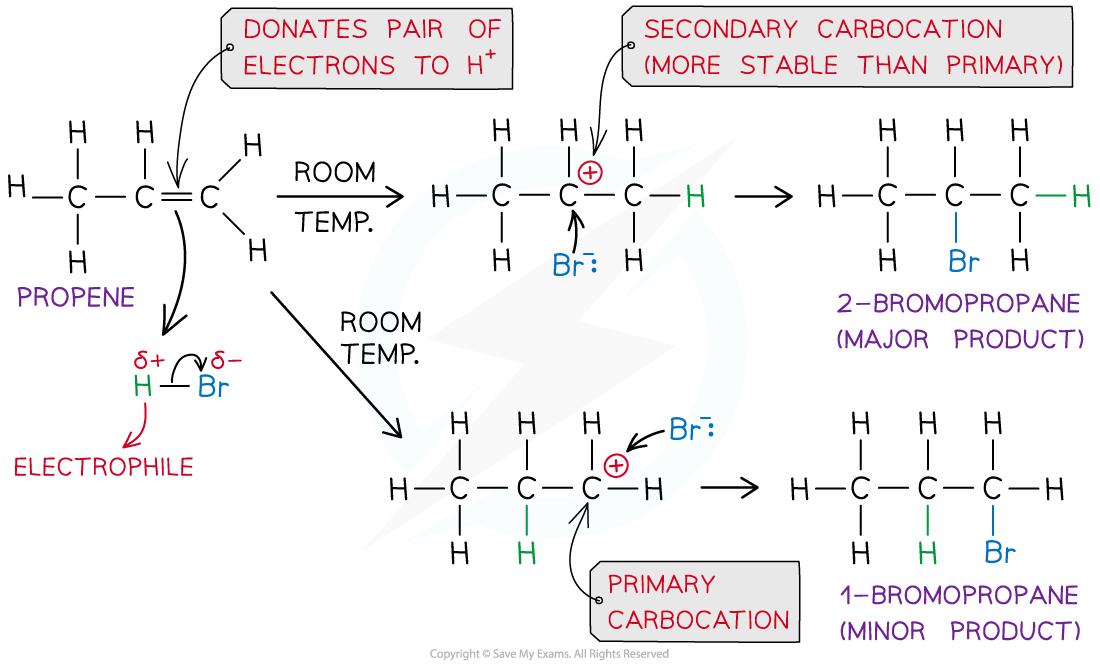
The electrophilic addition reaction mechanism of HBr and propene to form 1-bromopropane and 2-bromopropane
Exam Tip
The stability of the carbocation intermediate is as follows:
tertiary > secondary > primary
When more than one carbocation can be formed, the major product of the reaction will be the one that results from the nucleophilic attack of the most stable carbocation.

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









