- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记19.1.5 Free Energy & Eº
Free Energy & Eº
- Previously we have seen the concept and term free energy, ΔGθ
- Free energy is a measure of the available energy to do useful work and takes into account the entropy change, ΔSθ, as well as the enthalpy change of a reaction, ΔHθ
- For reactions to be spontaneous, the free energy change must be negative
- We have also seen that to calculate a cell potential using standard electrode potentials we use the expression:
EMF = ERHS – ELHS
- This is not an arbitrary arrangement of the terms
- The convention of placing the half cell with the greatest negative potential on the left of the cell diagram ensures that you will always get a positive reading on the voltmeter, corresponding to the spontaneous reaction
- If you have done an experiment on measuring electrode potentials, you have probably been told to 'swap the terminals if you don't get a positive reading on the voltmeter'
- In electrochemical cells, a spontaneous reaction occurs when the combination of half cells produces a positive voltage through the voltmeter, i.e. the more negative electrode pushes electrons onto the more positive electrode
- It doesn't really matter if you are using a digital multimeter as a voltmeter as you will still get a reading (with the wrong sign), but analogue voltmeters will only work if the terminals are correctly connected to the positive and negative half cells
- This should give you an insight into why the following statements are true:
If ΔEθ is positive, the reaction is spontaneous as written
If ΔEθ is negative, the forward reaction is non-spontaneous but the reverse reaction will be spontaneous
- You should now be able to see that there is a link between ΔGθ and Eθ
- This relationship is the equation:
ΔGθ = -nFEθ
where:
-
- n= number of electrons transferred
- F= the Faraday constant, 96 500 C mol-1
- When a reaction has reached equilibrium, there is no free energy change so ΔGθ is zero and it follows that Eθ must also be zero
- This is effectively what happens when the reactants in a voltaic cell have been exhausted and there is no longer any push of electrons from one half cell to the other
Worked Example
The spontaneous reaction between zinc and copper in a voltaic cell is shown below
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) Eθ cell = +1.10 V
Calculate the free energy change, ΔGθ, for the reaction.
Answer
-
- Write the equation:
ΔGθ =-nFEθ
-
- Substitute the values
ΔGθ = - 2 x 96 500 C mol-1 x 1.10 V =- 212300 C mol-1 V
-
- This looks a strange unit. However, by definition 1J = 1V x 1C, so this answer can be expressed as
ΔGθ = - 212300 J mol-1or -212.3 kJ mol-1
- The three conditions of free energy and electrode potential are summarised below
Summary table of the conditions of free energy and electrode potential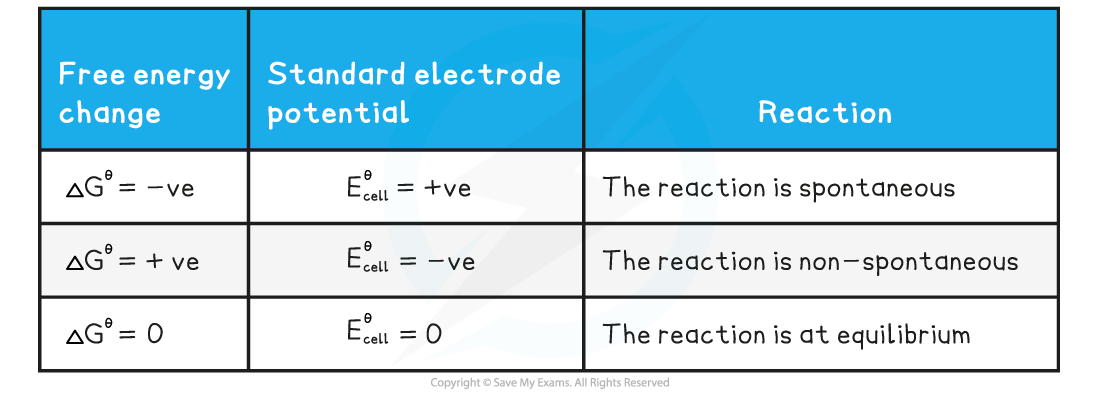
Exam Tip
The equation ΔGθ = -nFEθ is given in Section 1 of the Data Book so there is no need to memorise it
Predicting Spontaneous Reactions
- To predict the spontaneous reaction, we simply need to find the relevant half equations and electrode potentials
- From this information, we can deduce the spontaneous and non-spontaneous reaction
- By using the convention:
EMF = ERHS – ELHS
- A positive EMF is obtained from the spontaneous reaction which occurs when the most negative half cell is ELHS and the most positive is ERHS
- The left side is always where oxidation takes place so we can also us an alternative form of the relationship:
EMF = Ereduction – Eoxidation
Worked Example
Using data from Table 24 of the Data Book, determine if the reaction shown is spontaneous at standard conditions
Sn (s) + Mn2+ (aq) → Sn2+ (aq) + Mn (s)
Answer
-
- Table 24 of the Data Book shows the following half reactions
Sn2+ (aq) + 2e- → Sn (s) Eθ = -0.14 V
Mn2+ (aq) + 2e- → Mn (s) Eθ = -1.18 V
-
- Manganese is the more negative value, so will be ELHS or Eoxidation in the spontaneous reaction
EMF =ERHS – ELHS = (-0.14) - (-1.18) = +1.04
-
- For oxidation to take place, the manganese must lose electrons and the tin(II) must gain electrons
Mn (s) → Mn2+ (aq) + 2e- and Sn2+ (aq) + 2e- → Sn (s)
-
- So, the spontaneous reaction is
Mn (s) + Sn2+ (aq) → Mn2+ (aq) + Sn (s)
-
- Therefore, the reaction in the question is not spontaneous
Exam Tip
A word of cautionAlthough the positive Eθ indicates a reaction should take place, you might not actually see anything taking place if you constructed a cell that is predicted to be spontaneous. This is because like free energy changes, Eθ only predicts the energetic feasibility for a reaction and it does not take into account the rate of a reaction. A reaction could have a really high activation energy making it impossibly slow at room temperature.
'THERMODYNAMICS PREDICTS; KINETICS CONTROLS'
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









