- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记19.1.4 Quantitative Electrolysis
Quantitative Electrolysis
- Michael Faraday showed, in 1833, that the amount of products obtained is dependent on the quantity of electrical charge passed through the cell
- Electrical charge is a measure of the current passed and the time taken, and they are related in the following way:
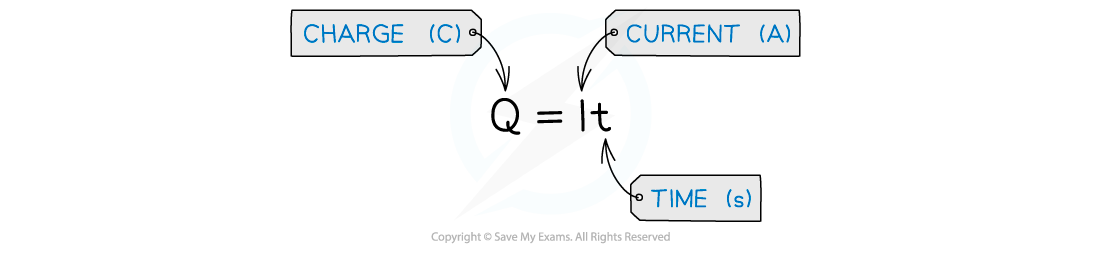
The formula for calculating charge
- The SI unit of electric current (symbol I) is the ampere, usually known as the amp (A).
- The SI unit of electric charge (symbol Q) is the coulomb (C)
- It is the amount of charge transported in 1 second by a current of 1 ampere
- The equation above can be written as:
Coulombs = Amps x seconds
C = A x s
- The charge on a single electron is 1.602 x 10-19 C, so one mole of electrons carries a charge of:
1.602 x 10-19 C x L = 1.602 x 10-19 x 6.02 x 1023 = 96 440.4 C mol-1
- This quantity is known as Faraday's constant or just the Faraday and is given the symbol F
- At this level for the purposes of calculations, this figure is rounded to 96 500 C mol-1
- To calculate the mass of a product at an electrode, knowing the current and time we follow these steps:
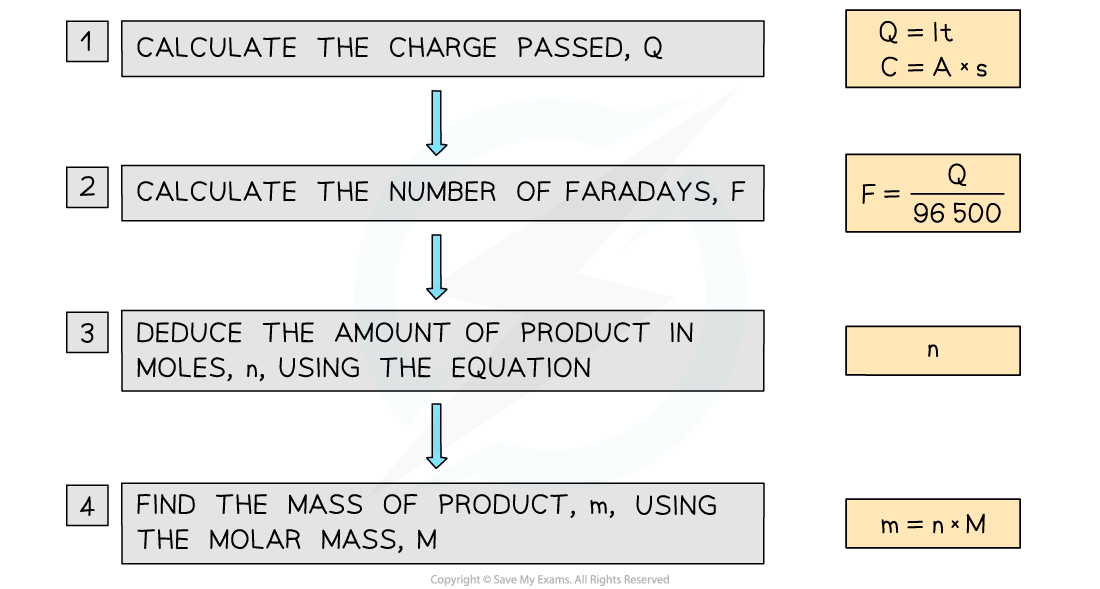
Solving problems in quantitative electrolysis
Worked Example
What mass of nickel is formed if a solution of nickel(II) sulfate is electrolysed for 10.0 mins using a current of 2.50 A?
Answer
-
- The amount of charge passed is:
Q = 2.50 x 10 x 60 = 1500 C
-
- The number of Faradays is:
F = 1500 ÷ 96 500 = 0.01554 mol
-
- The half equation is:
Ni2+ (aq) + 2e- → Ni (s)
-
- The mole ratio shows 2 moles electrons = 1 mole of Ni
- So, the amount of nickel is
n = 0.01554 mol ÷ 2 = 0.00777 mol
-
- The mass of nickel is
m = n x M = 0.00777 mol x 58.69 g mol-1 = 0.456 g
Relative amounts of products in electrolysis
- The half equations in electrolysis can be used to deduce the relative amounts of products at each electrode
- By looking at the amount of electrons needed to balance the half equations, we can deduce how much, in moles, of different products can be obtained by the same amount of charge passed
- Comparing the electrolysis of molten potassium chloride and lead chloride, the cathode reactions are:
K+ (l) + e- → K (l)
1 mole of electrons 1 mole of potassium
Pb2+ (l) + 2e- → Pb (s)
2 moles of electrons 1 mole of lead
- We can see that twice as many moles of electrons are needed to produce 1 mole of lead compared to 1 mole of potassium
- Alternatively, we can say that the same quantity of charge produces half as much lead as potassium (in moles)
Worked Example
A solution of silver nitrate, AgNO3, is electrolysed for 20 mins at a current of 1.50 A.1) How much silver, in moles, is formed at the cathode?2) How much copper, in moles, would be produced if the same reaction was carried out using copper(II) sulfate solution in place of silver nitrate?
Answer
Answer 1:
-
- The amount of charge passed is:
Q = 1.50 x 20 x 60 = 1800 C
-
- The number of Faradays is
F = 1800 ÷ 96 500 = 0.01865 mol
-
- The charge on the silver ion is +1, so 0.01865 mol of silver are produced
Answer 2:
-
- The half equations for silver and copper(II) are:
Ag+ (aq) + e- → Ag (s)
Cu2+ (aq) + 2e- → Cu (s)
-
- The same quantity of electrical charge will produce 2Ag: 1 Cu
- The amount of copper would be 0.01865 mol ÷ 2 = 0.009325 mol
Exam Tip
There is no need to learn the value of Faraday's constant as it is given in Section 2 of the Data booklet.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









