- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记19.1.3 Electrolysis of Aqueous Solutions
Electrolysis of Aqueous Solutions
- We have seen previously how simple binary compounds can be electrolysed when molten and the products of electrolysis can be predicted using our knowledge of the ions present
- At the cathode, positive metals ions (cations) are discharged resulting in metals being deposited
- The cations are reduced by the electrons coming from the cathode:
Pb2+(l) + 2e- → Pb (l)
- Meanwhile, at the anode, anions are discharged by oxidation:
2Br- (l) → Br2 (g) + 2e-
- However, when aqueous solutions of ionic compounds are electrolysed the products are a little more complicated to predict as there are additional ions present from the water
- Water can be oxidised to oxygen or reduced to hydrogen:
- Oxidation reaction:
2H2O (l) → 4H+ (aq) + O2 (g) + 4e-
-
- Reduction reaction:
2H2O (l) + 2e-→ H2 (g) + 2OH- (aq)
- At the cathode, either the metal ion M+ or water can be reduced
- At the anode, either the anion A- or water can be oxidized
- Which species is discharged depends on three things:
- The relative values of Eθ
- The concentration of the ions present
- The identity of the electrode
Products of specified electrolytes
- The electrolysis of water, sodium chloride solution and copper sulfate solutions is as follows:
Table showing the electrolysis products of aqueous solutions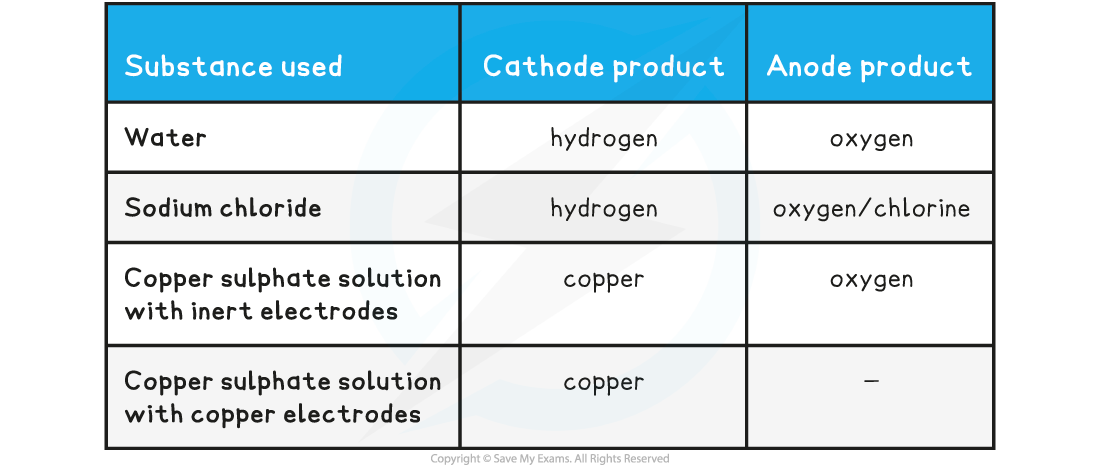
The influence of relative values of Eθ
- The electrolysis of water is very slow as there are few ions present, so a little acid or base can be added to increase the number of ions present and speed up the electrolysis
- Whether acid or base is added the products are the same, but the electrode reactions are slightly different
- Using dilute sulfuric acid as the electrolyte, the cathode reactions could be
2H2O (l) + 2e- → H2 (g) + 2OH- (aq) Eθ = -0.83V
2H+ (aq) + 2e- → H2 (g) Eθ = 0.00 V
- The Eθ is smaller for the hydrogen ion so it is preferentially reduced and H2 (g) will be discharged
- At the anode, although sulfate ions are present in the solution, only water can be oxidised
- This is because the sulfate ion, SO42-, contains sulfur in its maximum oxidation state (+6) so it cannot be further oxidised
- The oxidation of water produces oxygen gas:
2H2O (l) → 4H+ (aq) + O2 (g) + 4e- Eθ = -1.23 V
- If the water is made basic by the addition of dilute sodium hydroxide solution, the cathode reactions could be:
Na+ (aq) + e- → Na (s) Eθ = -2.71 V
2H2O (l) + 2e- → H2 (g) + 2OH- (aq) Eθ = -0.83 V
- The Eθ is smaller for water than the sodium ion, so water is preferentially reduced and H2 (g) will be discharged
- At the anode, either the hydroxide ion or water can be oxidised:
4OH- (aq) → 2H2O (l) + O2 (g) + 4e- Eθ = -0.40 V
2H2O (l) → 4H+ (aq) + O2 (g) + 4e- Eθ = -1.23 V
- Based on these values the hydroxide ion is preferentially oxidized and O2 (g) will be discharged
- The overall reaction whether in acid or alkali conditions is:
2H2O (l) → 2H2 (g) + O2 (g)
The influence of concentration of the ions
- The electrolysis of sodium chloride solution provides an illustration of the influence of concentration on the products discharged
- As before, we would expect hydrogen ion to be preferentially discharged at the cathode before the sodium ion:
2H+ (aq) + 2e- → H2 (g) Eθ = 0.00 V
- However at the anode, the relative proximity of the Eθ values allows the possibility of both reactions occurring:
2Cl- (aq) → Cl2 (g) + 2e- Eθ = -1.36 V
2H2O (l) → 4H+ (aq) + O2 (g) + 4e- Eθ = -1.23 V
- In fact, when concentration of the sodium chloride increases to more than 25% the Cl- becomes preferentially discharged and chlorine gas is the main product of the reaction at the anode
- The overall reaction equation is:
2NaCl (aq) + 2H2O (l) → 2NaOH (aq) + H2 (g) + Cl2 (g)
Influence of the electrodes
- The products of electrolysis are influenced by the identity of the electrodes
- Electrodes that take part in the redox processes are know as active electrodes and inert electrodes such as platinum and carbon are called passive electrodes
- The electrolysis of copper sulfate solution, CuSO4 (aq), is an example of where active and passive electrodes determine the products
Active electrodes
- At the cathode, the possible reactions that could take place are:
Cu2+ (aq) + 2e- → Cu (s) Eθ = +0.34 V
2H2O (l) + 2e- → H2 (g) + 4OH- (aq) Eθ = -0.83 V
- Copper ions are preferentially reduced, so copper metal is deposited on the cathode
- At the anode, water is oxidised, so oxygen gas is produced (the sulfate ion cannot be oxidised):
2H2O (l) → 4H+ (aq) + O2 (g) + 4e- Eθ = -1.23 V
- The overall equation for the reaction is:
2CuSO4 (aq) + 2H2O (l) → 2Cu (s) + O2 (g) + 2SO42- (aq) + 4H+ (aq)
OR
2CuSO4 (aq) + 2H2O (l) → 2Cu (s) + O2 (g) + 2H2SO4 (aq)
Passive electrodes
- At the cathode, the reaction is the same as with inert electrodes:
Cu2+ (aq) + 2e- → Cu (s) Eθ = +0.34 V
- However, at the anode the copper electrode is oxidised and dissolves to form copper ions
Cu (s) → Cu2+ (aq) + 2e- Eθ = -0.34 V
- This reaction is used to purify copper, needed to produce a very high grade of copper for use in electrical wires
- The impure copper is made the anode, and the cathode is made of pure copper
- The impurities from the anode fall to the bottom of the cell
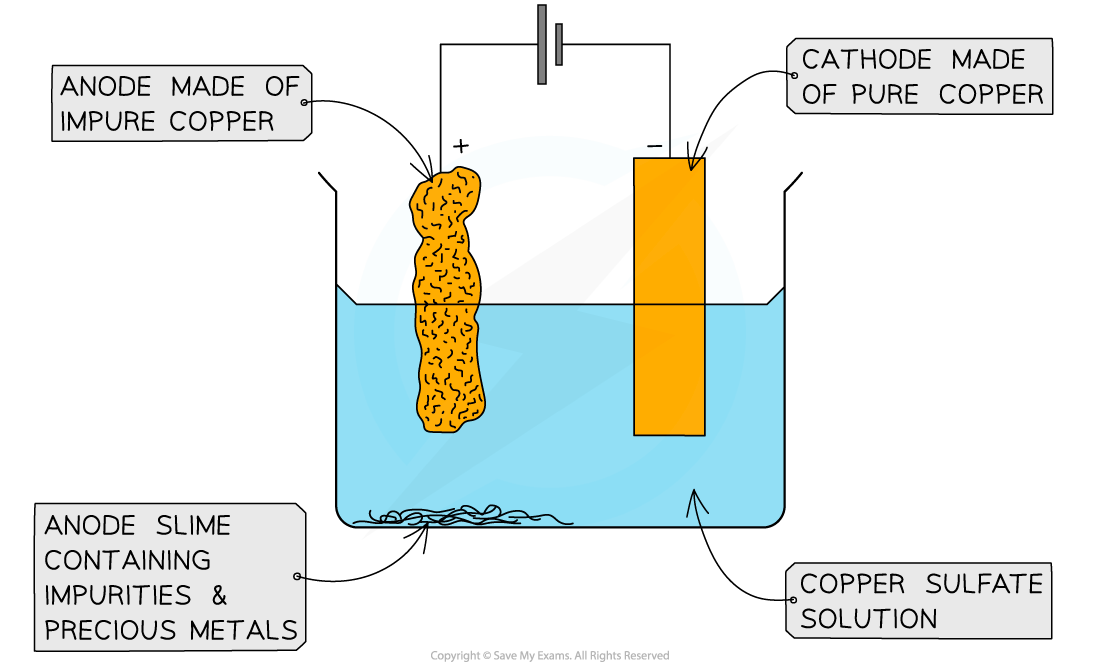
The purification of copper by electrolysis
- The anode slowly dissolves away and the cathode builds up pure copper
- The impurities form a slime under the anode which is actually quite valuable as it often contains significant quantities of precious metals like silver
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








