- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记19.1.2 The Standard Hydrogen Electrode
The Standard Hydrogen Electrode
- The absolute value of a half cell potential cannot be measured, only differences in potential between pairs of half-cells.
- For this reason, it is necessary to have a standard electrode against which all other half-cells can be compared
- The standard hydrogen electrode is a half-cell used as a reference electrode and consists of:
- Hydrogen gas in equilibrium with H+ ions of concentration 1.00 mol dm-3 (at 100 kPa)
2H+ (aq) + 2e– ⇌ H2 (g)
-
- An inert platinum electrode that is in contact with the hydrogen gas and H+ ions
- It is given an arbitrary value of Eθ = 0.00 volts
- When the standard hydrogen electrode is connected to another half-cell, the standard electrode potential of that half-cell can be read from a high resistance voltmeter
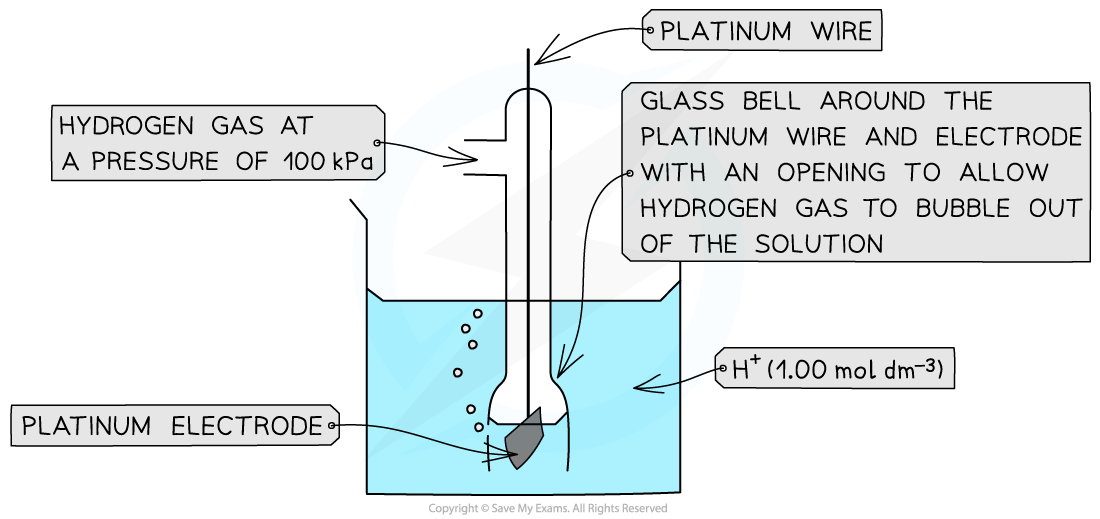 The standard electrode potential of a half-cell can be determined by connecting it to a standard hydrogen electrode
The standard electrode potential of a half-cell can be determined by connecting it to a standard hydrogen electrode
- In fact, the hydrogen electrode is rarely used in practice for a number of reasons:
- The electrode reaction is slow
- The electrodes are not easily portable
- It is difficult to maintain a constant pressure
- Once one standard electrode potential has been measured relative to the standard hydrogen electrode, it is not necessary to use the standard hydrogen electrode again
- Any electrode whose electrode potential is known could be used to measure standard electrode potentials
Measurements using the hydrogen electrode
- If a hydrogen electrode is used to measure the electrode potentials of zinc and copper half reactions, the conventional cell diagrams would be:
Pt 丨H2(g), 2H+(aq) ∥ Zn2+ (aq), Zn (s) Eθ = -0.76 V
Pt 丨H2(g), 2H+(aq) ∥ Cu2+ (aq), Cu (s) Eθ = +0.34 V
- Since the hydrogen electrode is always on the left, the polarity of the half cell measured is always with respect to hydrogen
- The half reaction will therefore always be a reduction reaction, so that is why sometimes standard electrode potentials are termed standard reduction potentials
- Tables of standard electrode potentials have been compiled ranking half cells from negative to positive values
Table of standard electrode potentials

- The more negative the value; the better the half cell is at pushing electrons so the equilibrium lies to the left
- This means the more negative the half cell; the better it can act as a reducing agent
Exam Tip
You might find this a helpful mnemonic for remembering the redox processes in cells
Lio the lion goes Roor!
Lio stands for ‘Left Is Oxidation’ and he is saying ROOR because that is the order of species in the cell diagram:Reduced 丨 Oxidised ∥ Oxidised丨Reduced
Pt 丨Fe2+(aq), Fe3+(aq) ∥ Cl2 (g), 2Cl- (aq) 丨Pt
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









