Weak acids
- A weak acid is an acid that partially (or incompletely) dissociates in aqueous solutions
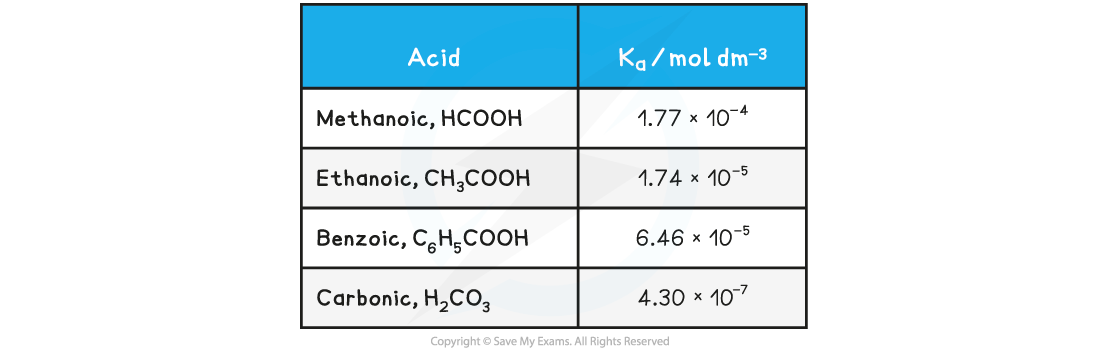
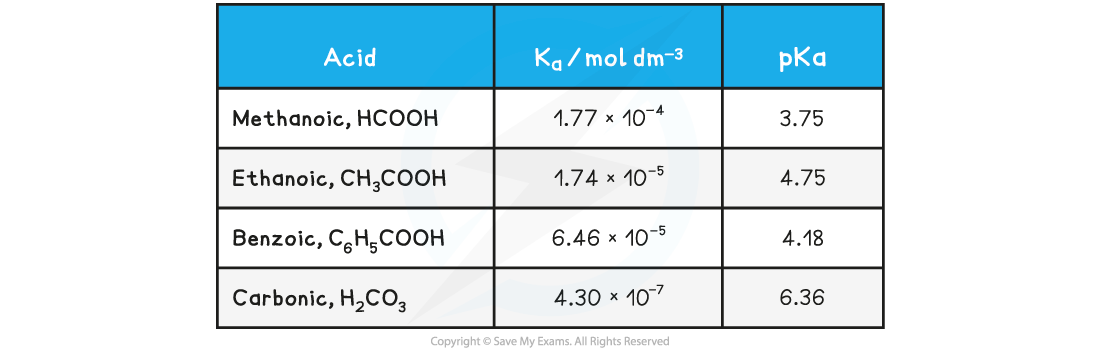
- For example, most carboxylic acids (e.g. ethanoic acid), HCN (hydrocyanic acid), H2S (hydrogen sulfide) and H2CO3 (carbonic acid)
- In general, the following equilibrium is established:
HA (aq) + H2O (l) ⇌ A- (aq) + H3O+ (aq)
OR
HA (aq) ⇌ A- (aq) + H+ (aq)