- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记17.1.2 Gibbs Free Energy & the Equilibrium Constant
Gibbs Free Energy & the Equilibrium Constant
Gibbs Free Energy & the Equilibrium Constant
- The equilibrium constant, Kc, gives no information about the individual rates of reaction
- It is independent of the kinetics of the reaction
- The equilibrium constant, Kc, is directly related to the Gibbs free energy change, ΔGꝊ, according to the following (van't Hoff's) equation:
ΔGꝊ = -RT lnK
-
- ΔGꝊ= Gibbs free energy change (kJ mol–1)
- R = gas constant (8.31 J K-1 mol-1)
- T = temperature (Kelvin, K)
- K = equilibrium constant
- This equation is provided in section 1 of the data booklet
Exam Tip
When completing calculations using the ΔGꝊ = -RT lnK equation, you have to be aware that:
- ΔGꝊ is measure in kJ mol–1
- R is measured in J K-1 mol-1
This means that one of these values will need adjusting by a factor of 1000
- This relationship between the equilibrium constant, Kc, and Gibbs free energy change, ΔGꝊ, can be used to determine whether the forward or backward reaction is favoured
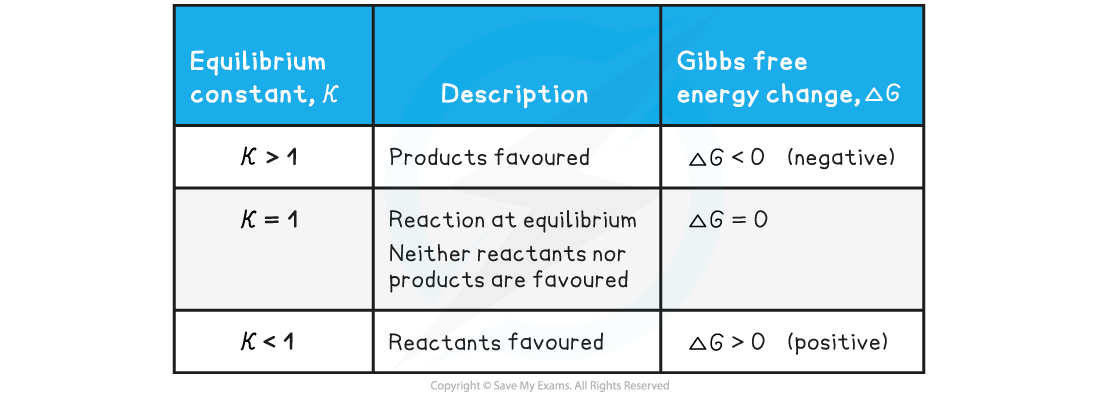
The relationship between the equilibrium constant, Kc, and Gibbs free energy change, ΔGꝊ
- At a given temperature, a negative ΔG value for a reaction indicates that:
- The reaction is feasible / spontaneous
- The equilibrium concentration of the products is greater than the equilibrium concentration of the reactants
- The value of the equilibrium constant is greater than 1
- As ΔG becomes more negative:
- The forward reaction is favoured more
- The value of the equilibrium constant increases
Free Energy & Equilibrium Calculations
- The relationship between Gibbs free energy change, ΔGꝊ, temperature and the equilibrium constant, Kc, is described by the equation:
ΔGꝊ = -RT lnK
- The rearrangement of this equation makes it possible to:
- Calculate the equilibrium constant
- Deduce the position of equilibrium for the reaction
Worked Example
Calculating KcEthanoic acid and ethanol react to form the ester ethyl ethanoate and water as follows:
CH3COOH (I) + C2H5OH (I) ⇌ CH3COOC2H5 (I) + H2O (I)
At 25 oC, the free energy change, ΔGꝊ, for the reaction is -4.38 kJ mol-1. (R = 8.31 J K-1 mol-1)
- Calculate the value of Kc for this reaction
- Using your answer to part (1), predict and explain the position of the equilibrium
Answers
Answer 1:
Step 1: Convert any necessary values
-
- ΔGꝊ into J mol-1:
- -4.38 x 1000 = -4380 J mol-1
- T into Kelvin
- 25 + 273 = 298 K
- ΔGꝊ into J mol-1:
Step 2: Write the equation:
-
- ΔGꝊ = -RT lnKc
Step 3: Substitute the values:
-
- -4380 = -8.31 x 298 x lnKc
Step 4: Rearrange and solve the equation for Kc:
-
- lnKc = -4380 ÷ (-8.31 x 298)
- lnKc = 1.77
- Kc = e1.77
- Kc = 5.87
Answer 2:
From part (1), the value of Kc is 5.87
Therefore, the equilibrium lies to the right / products side because the value of Kc is positive
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









