- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记15.2.5 Free Energy & Equilibrium
Free Energy & Equilibrium
- When ΔG < 0 for a reaction at constant temperature and pressure, the reaction is spontaneous
- When a reversible reaction reaches equilibrium, the Gibbs free energy is changing as the ratio of reactants to products changes
- For non-reversible reactions:
- As the amount of products increases, the reaction moves towards completion
- This leads to a decrease in Gibbs free energy
- For reversible reactions:
- As the amount of products increases, the reaction moves towards equilibrium
- This causes a decrease in Gibbs free energy
- At the point of equilibrium, Gibbs free energy is at its lowest as shown on the graph:
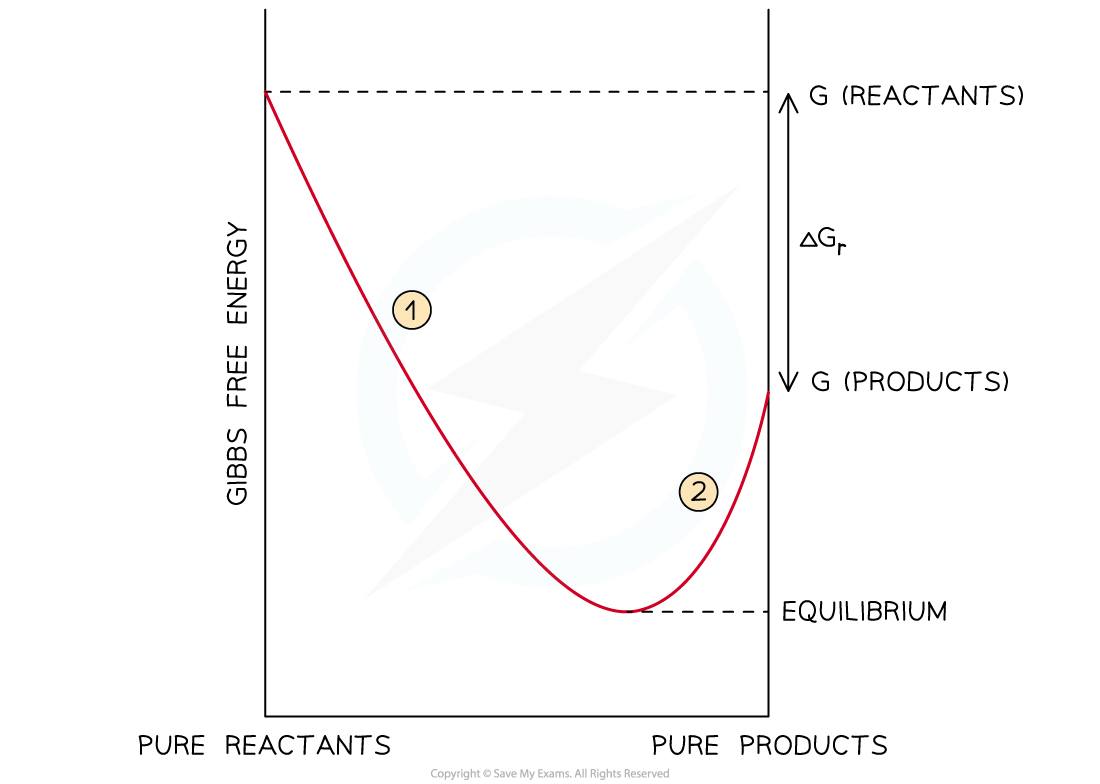
Gibbs free energy changes as the reaction proceeds
- In section 1 of the graph, the forward reaction is favoured and the reaction proceeds towards a minimum value
- Having reached a point of equilibrium, the Gibbs free energy increases
- This is when the reaction becomes non-spontaneous (section 2)
- The reverse reaction now becomes spontaneous and the Gibbs free energy again reaches the minimum value, so heads back towards equilibrium
- The reaction will be spontaneous in the direction that results in a decrease in free energy (becomes more negative)
- When the equilibrium constant, K, is determined for a given reaction, its value indicates whether the products or reactants are favoured at equilibrium
- ΔG is an indication of whether the forward or backward reaction is favoured
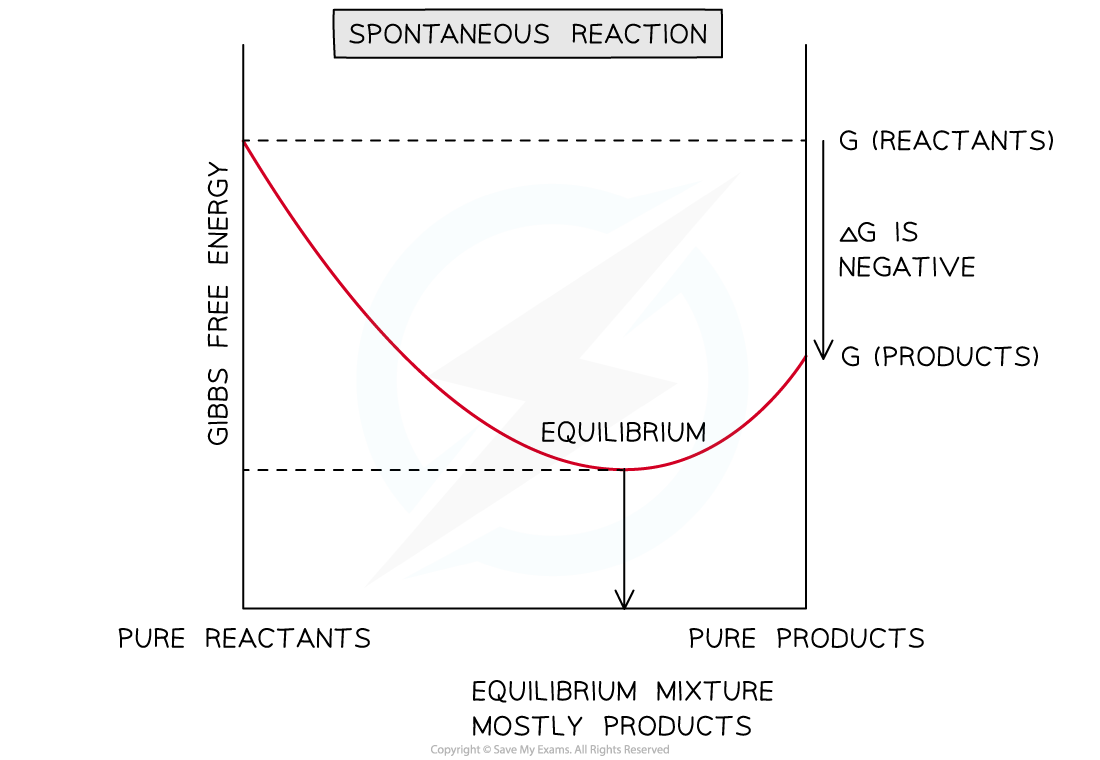
Graph for a spontaneous reaction
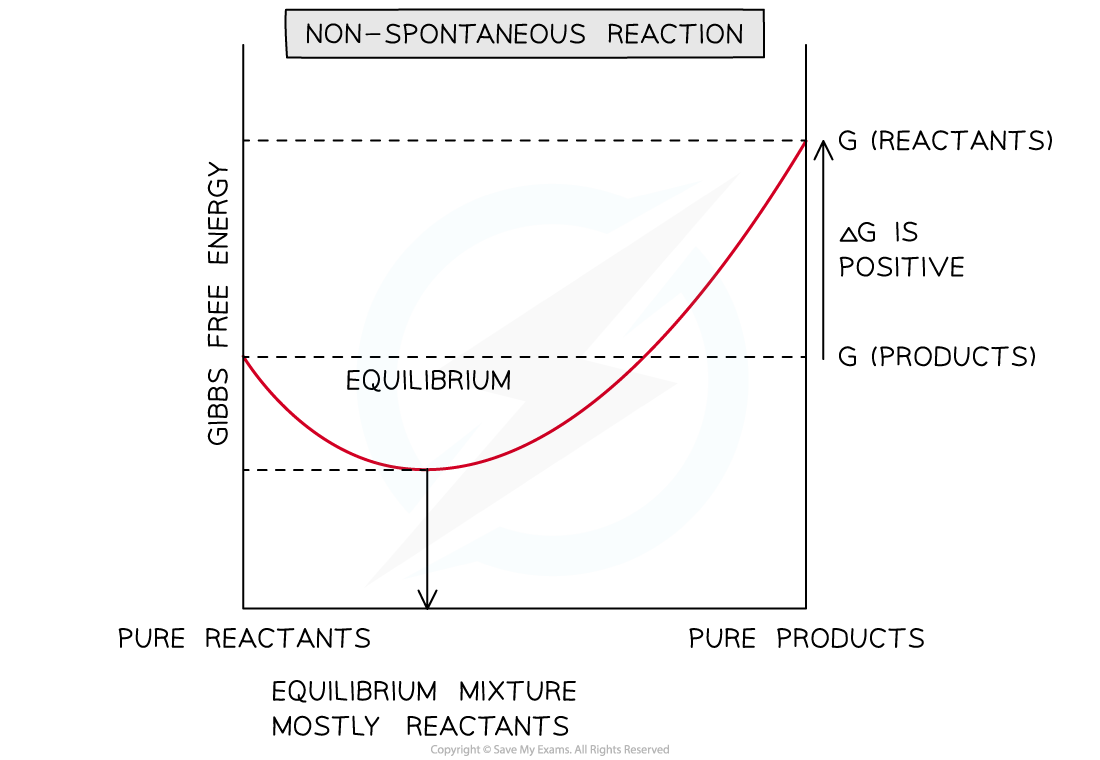
Graph for a non-spontaneous reaction
- The quantitative relationship between standard Gibbs free energy change, temperature and the equilibrium constant is represented by:
ΔGꝋ = -RT In K
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








