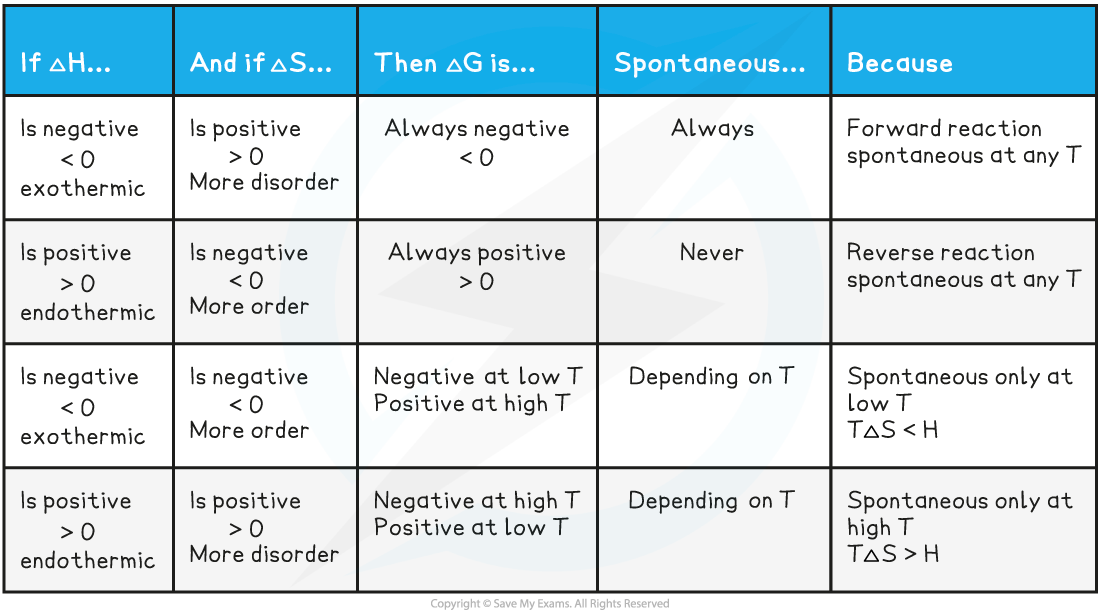
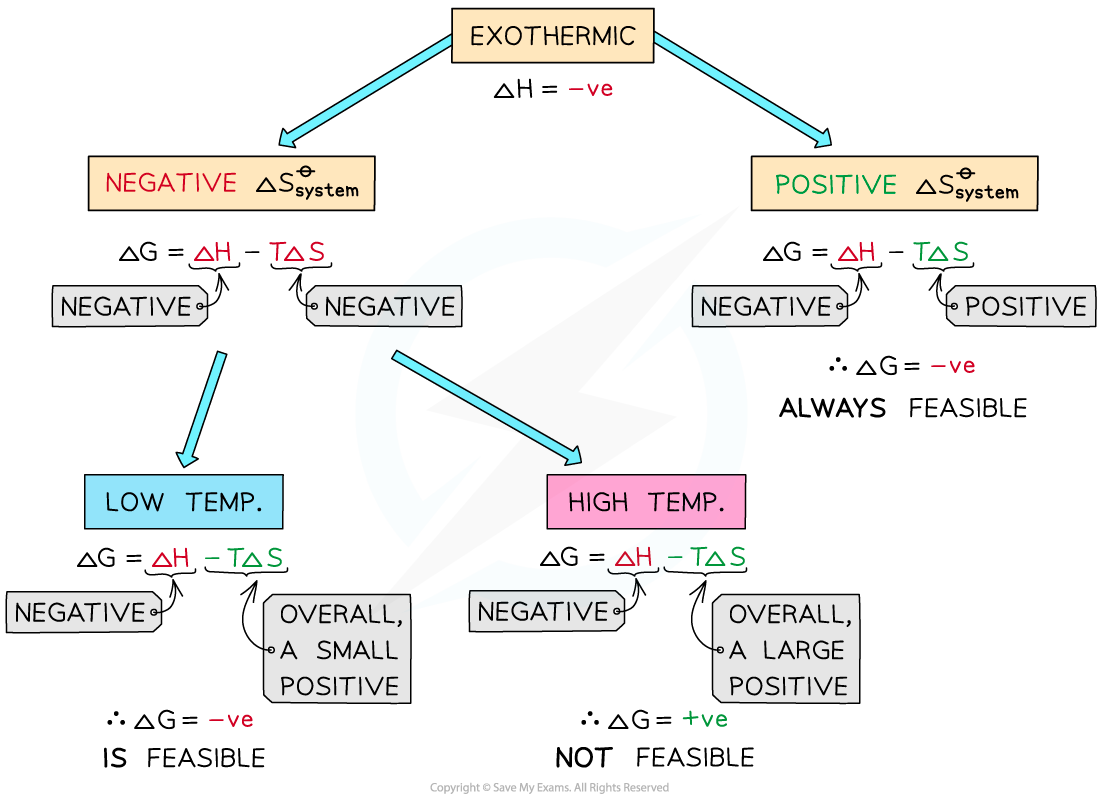
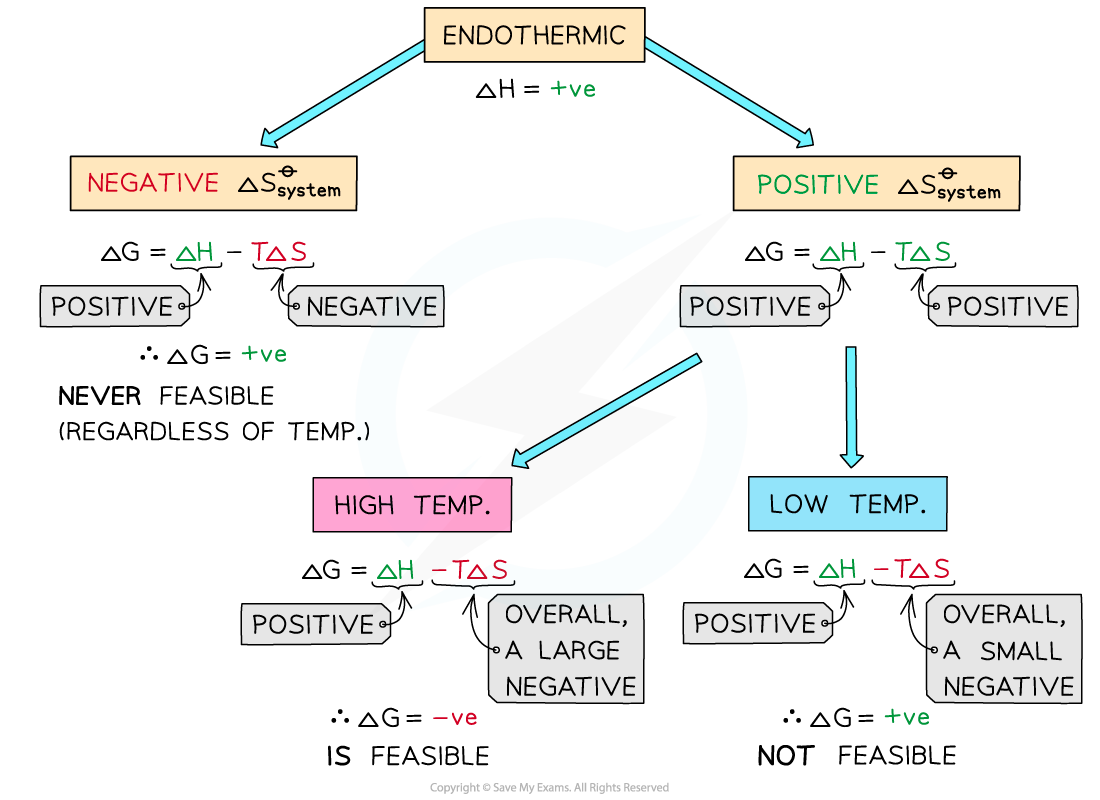
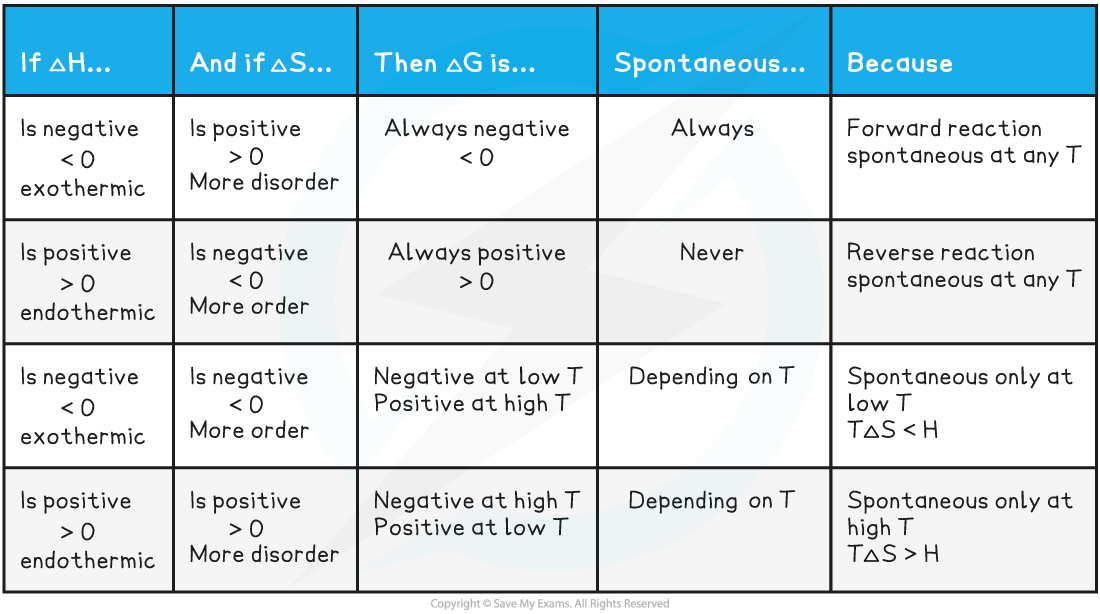
Endothermic reactions
- In endothermic reactions, ΔHreactionꝋ is positive
- If the ΔSsystemꝋ is negative:
- Both the first and second term will be positive
- Resulting in a positive ΔGꝋ so the reaction is not feasible
- Therefore, regardless of the temperature, endothermic with a negative ΔSsystemꝋ will never be feasible
- If the ΔSsystemꝋ is positive:
- The first term is positive and the second term is negative
- At low temperatures, the –TΔSsystemꝋ will be small and negative and will not overcome the larger ΔHreactionꝋ
- Therefore, at low temperatures ΔGꝋ is positive and the reaction is not feasible
- The reaction is more feasible at high temperatures as the second term will become negative enough to overcome the ΔHreactionꝋ resulting in a negative ΔGꝋ
- This tells us that for certain reactions which are not feasible at room temperature, they can become feasible at higher temperatures
- An example of this is found in metal extractions, such as the extraction if iron in the blast furnace, which will be unsuccessful at low temperatures but can occur at higher temperatures (~1500 oC in the case of iron)