- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记15.2.3 Gibbs Free Energy
Gibbs Free Energy Change
Gibbs free energy
- The feasibility of a reaction is determined by two factors, the enthalpy change and the [popover id="F7rfknVfbfcSJVkN" label='entropy change']
- The two factors come together in a fundamental thermodynamic concept called the Gibbs free energy (G)
- The Gibbs equation is:
ΔGꝋ = ΔHreactionꝋ – TΔSsystemꝋ
-
- The units of ΔGꝋ are in kJ mol–1
- The units of ΔHreactionꝋ are in kJ mol–1
- The units of T are in K
- The units of ΔSsystemꝋ are in J K-1 mol–1(and must therefore be converted to kJ K–1 mol–1 by dividing by 1000)
Calculating ΔGꝋ
- There are two ways you can calculate the value of ΔGꝋ
- From the Gibbs equation, using enthalpy change, ΔHꝋ, and entropy change, ΔSꝋ, values
- From ΔGꝋ values of all the substances present
Calculating Delta G from the Gibbs Equation
Worked Example
ΔGꝋ from ΔHꝋ and ΔSꝋ valuesCalculate the free energy change for the following reaction:
2NaHCO3 (s) → Na2CO3 (s) + H2O (l) + CO2 (g)
- ΔHꝋ = +135 kJ mol-1
- ΔSꝋ = +344 J K-1 mol-1
Answer:
Step 1: Convert the entropy value in kilojoules
-
-
- ΔSꝋ = +344 J K-1 mol-1 ÷ 1000 = +0.344 kJ K-1 mol-1
-
Step 2: Substitute the terms into the Gibbs Equation
-
-
- ΔGꝋ = ΔHreactionꝋ – TΔSsystemꝋ
- = +135 – (298 x 0.344)
- = +32.49 kJ mol-1
- ΔGꝋ = ΔHreactionꝋ – TΔSsystemꝋ
-
The temperature is 298 K since standard values are quoted in the question
Calculating Delta G from Delta G Formation
Worked Example
ΔGꝋ from other ΔGꝋ valuesWhat is the standard free energy change, ΔGꝋ, for the following reaction?
C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(g)
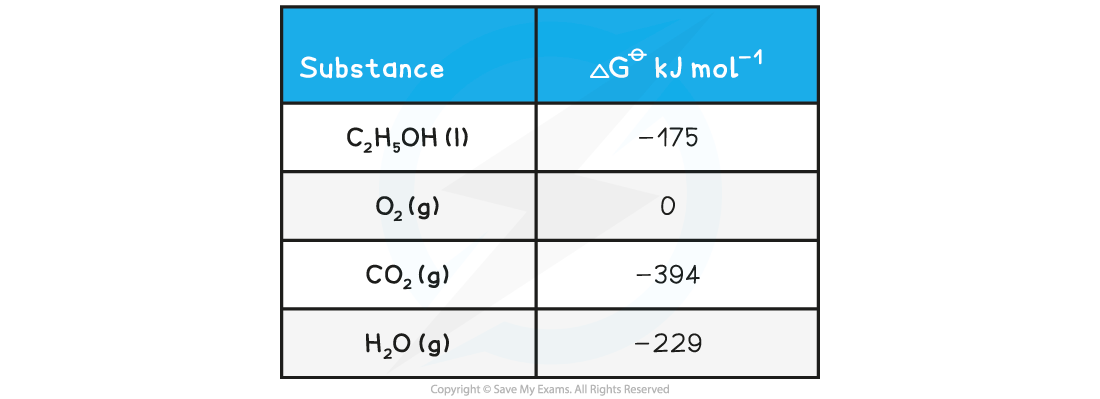
Answer:
-
- This can be calculated in the same way as you complete enthalpy calculations
-
- ΔGꝋ = [(2 x CO2 ) + (3 x H2O )] – [(C2H5OH) + (3 x O2)]
- ΔGꝋ = [(2 x -394) + (3 x -229 )] – [-175 + 0]
- ΔGꝋ = -1300 kJ mol-1 ΔGꝋ = ΣΔGproductsꝋ – ΣΔGreactantsꝋ
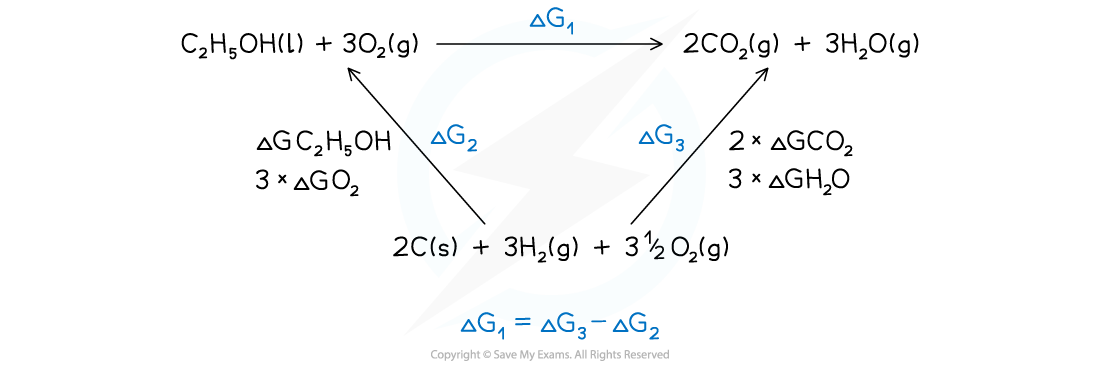
- This can also be done by drawing a Hess cycle - find the way that is best for you
Exam Tip
The idea of free energy is what’s ‘leftover’ to do useful work when you’ve carried out the reaction.The enthalpy change is the difference between the energy you put in to break the chemical bonds and the energy out when making new bondsThe entropy change is the ‘cost’ of carrying our the reaction, so free energy is what you are left with!
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








