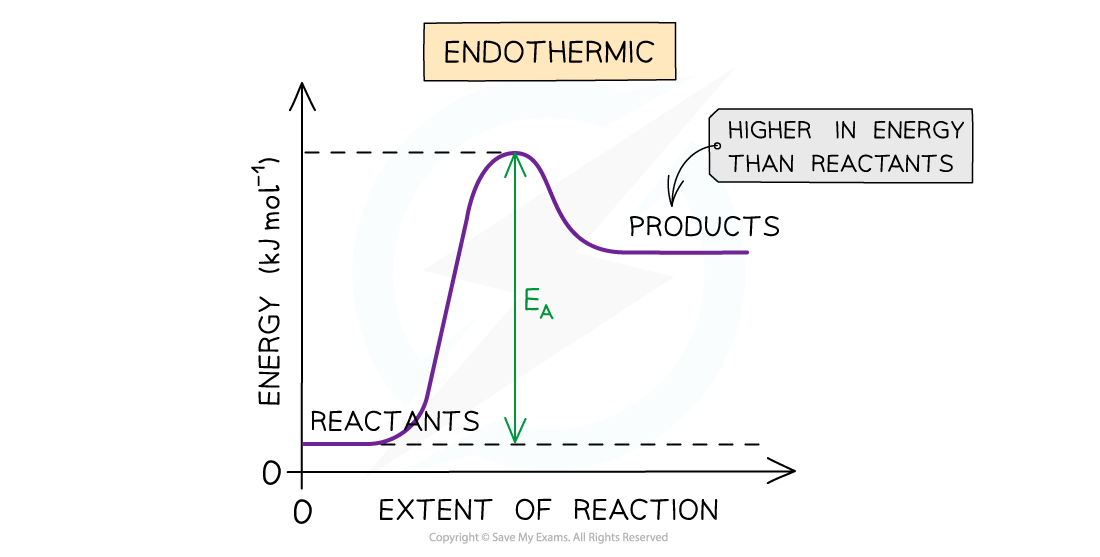
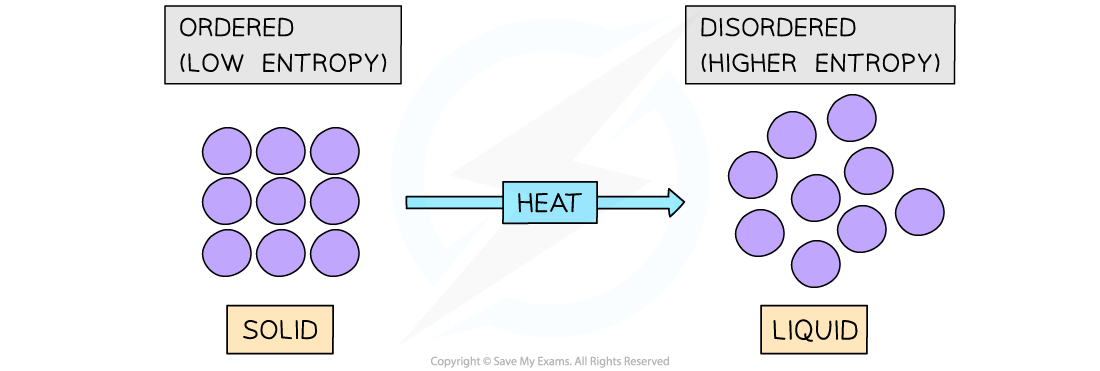
- The entropy (S) of a given system is the number of possible arrangements of the particles and their energy in a given system
- In other words, it is a measure of how disordered or chaotic a system is
- When a system becomes more disordered, its entropy will increase
- An increase in entropy means that the system becomes energetically more stable
- For example, during the thermal decomposition of calcium carbonate (CaCO3) the entropy of the system increases:
CaCO3(s) → CaO(s) + CO2(g)
-
- In this decomposition reaction, a gas molecule (CO2) is formed
- The CO2 gas molecule is more disordered than the solid reactant (CaCO3), as it is constantly moving around
- As a result, the system has become more disordered and there is an increase in entropy