- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记15.1.5 Energy Changes in Aqueous Solutions
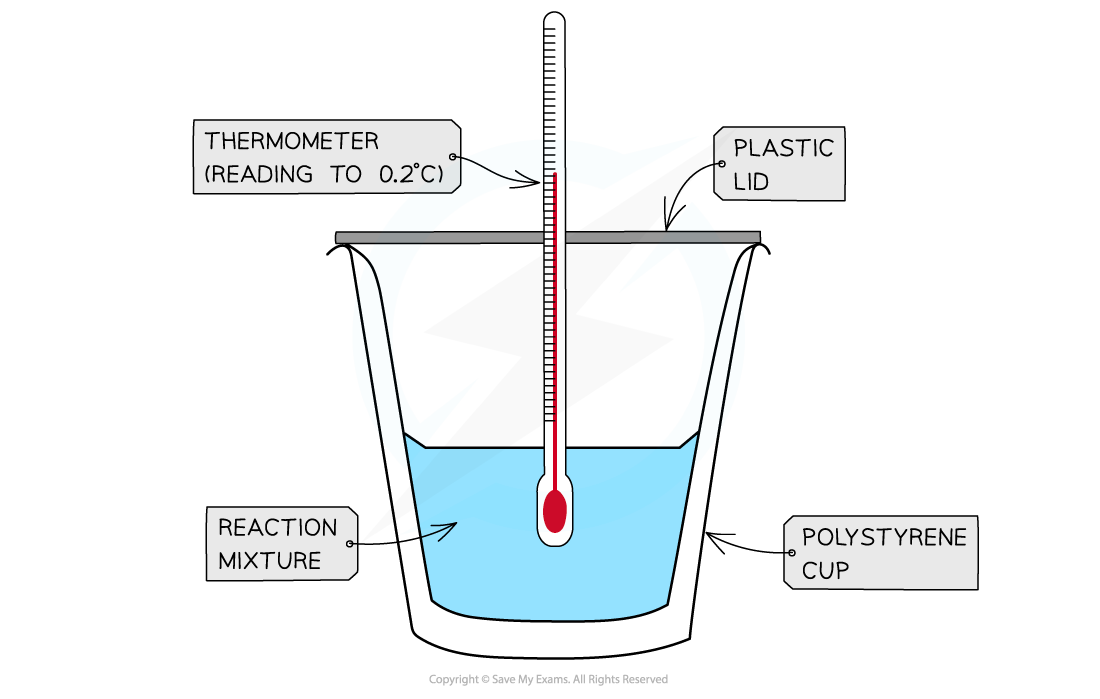
Measuring Energy Changes in the Lab
Enthalpies of Solution
- Calorimetry can be used to find the energy change in chemical reactions.
- This is much easier to carry out in aqueous solutions
- We can take different ionic compounds in the solid phase and dissolve them in water to determine lattice enthalpy
- We must know:
- The mass of water used
- The mass of solid used, so recording the mass of the weighing vessel should be taken before and after
- The temperature of the water should be recorded for a minimum period of 2 minutes before the solid is added
- Once the solid is added then the temperature can be recorded every 30 seconds for 10 minutes
- If the reaction is exothermic, the temperature will increase
- If the reaction is endothermic, the temperature will decrease
- The highest or lowest temperature recorded will be used to measure the difference in temperature
- For example, if the initial temperature of the water is 22.4 oC and the lowest temperature recorded was 12.6 oC the temperature change would be 9.8 oC
- The energy needed to increase the temperature of 1 g of a substance by 1 oC is called the specific heat capacity (c) of the liquid
- The specific heat capacity of water is given in the data booklet as 4.18 J g-1 K-1
- The energy transferred as heat can be calculated by:
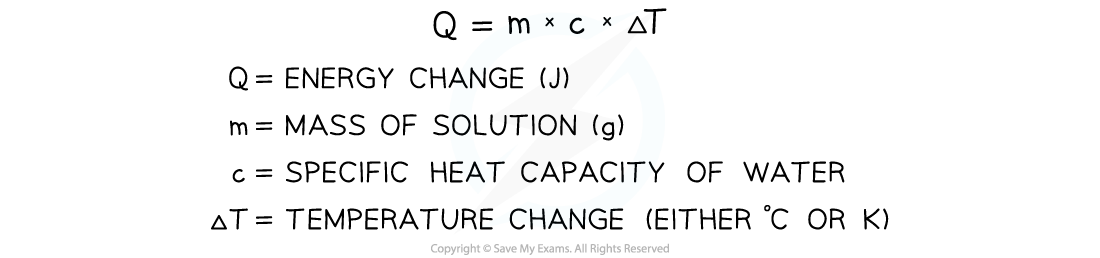
Equation for calculating energy transferred in a calorimeter
- Once we know Q from the above equation, we can calculate the enthalpy change for the reaction
- Enthalpy of solution can be calculated by working out the energy change per mole of compound
- Remember, in the equation temperature change is required, i.e. the difference between the first reading and the highest / lowest reading
- Therefore, the units for temperature do not matter as the change will still be the same in oC or K
Experiment 1: Addition of Ammonium Chloride and Sodium Iodide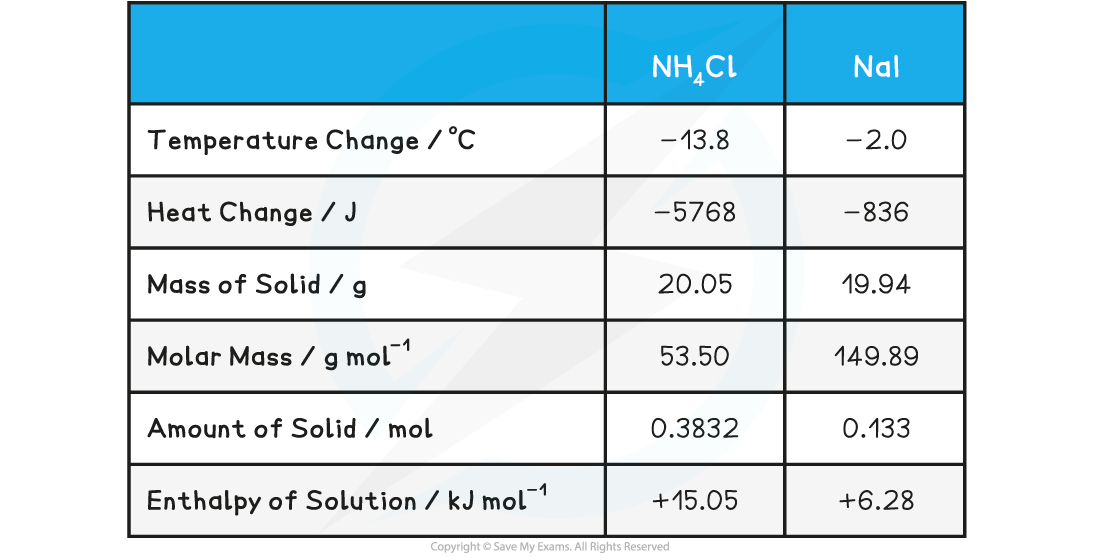
Sample data for the reaction of ammonium chloride, NH4Cl, and sodium iodide, NaI, with water
To calculate an experimental value for enthalpy change of the reaction, ΔH, we can follow the same basic steps:
- Step 1: Compile the relevant important information:
- Mass of water used in experiment = 100 g
- Temperature change = 13.8 oC (endothermic reaction)
- Mass of NH4Cl = 20.05 g
- Molar mass of NH4Cl = 53.50 g mol-1
- Step 2: Calculate the energy change for the reaction:
- Q = mcΔT
- Q = 100 x 4.18 x 13.8
- Q = 5768 J
- Q = mcΔT
- Step 3: Calculate the number of moles of NH4Cl
- Moles = mass / molar mass
- Moles of NH4Cl = 20.05 / 53.50
- Moles of NH4Cl = 0.3747 moles
- Moles = mass / molar mass
- Step 4: Calculate the energy change per mole of compound:
- ΔH = Q / moles
- ΔH = 5768 / 0.3747
- ΔH = 15390.9226 J mol-1
- ΔH = Q / moles
- Therefore, in kJ mol-1, the enthalpy change, ΔH, is +15.39 kJ mol-1
- The data book value for the enthalpy of solution, ΔHsolꝋ of NH4Cl is +14.78 kJ mol-1
- The same calculations can be done for sodium iodide
Errors in this method
- Errors in the method will lead to a difference in the data book value and the experimental value
- For this method, some of these errors are:
- Energy transfer to the surroundings (usually loss)
- This is the largest and most obvious error
- Approximation in specific heat capacity of water
- This method assumes all solutions have the heat capacity of water
- Neglecting the specific heat capacity of the calorimeter
- The method ignores that the apparatus will absorb energy
- An incomplete or slow reaction
- Density of the solution is taken to be the same as water
- Energy transfer to the surroundings (usually loss)
- A data logger could be used in this method to record the temperature for this method which would considerably reduce the source of uncertainty
- Some solids also will absorb moisture from the atmosphere
- This partly hydrates the solid and also effects the molar mass of the compound
- Data book values will refer to 'infinite dilution' whereas the solutions produced in this method are quite concentrated
Experiment 2: Addition of Zinc Powder to Copper(II) Sulfate
- The same calorimetry method can be used to record the temperature changes and therefore the ΔH for the reaction
- A known mass of zinc, Zn, is added to 50 cm3 of 1.00 mol dm-3 copper(II) sulfate solution, CuSO4 (aq), and the temperature is recorded every 30 seconds for at least 10 minutes
- A graph can be drawn from the data which shows a maximum recorded temperature of ΔT = 35.2 oC
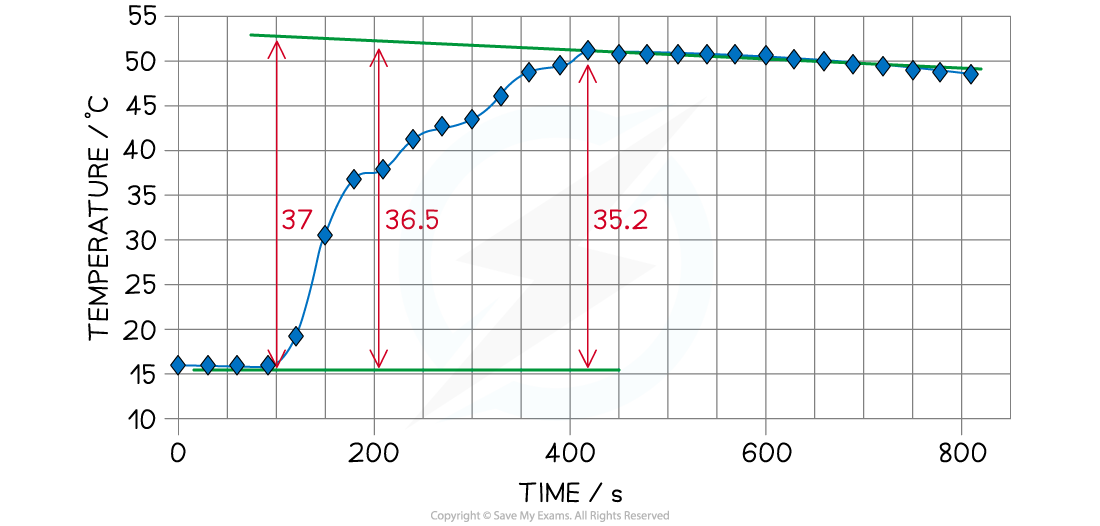
Temperature change for the reaction of zinc with copper(II) sulfate
- However, we need to extrapolate the graph to when the zinc was added which gives ΔT as roughly 37 oC
- Important information
- Volume of 1.00 mol dm-3 CuSO4 used in experiment = 50 cm3
- Temperature change = 37 oC (exothermic reaction)
- Mass of weighing bottle and Zn = 6.087 g
- Mass of weighing bottle after emptying = 1.064 g
- Molar mass of Zn = 65.38 g mol-1
- For this experiment, it is slightly different as there must be a limiting reagent
- In order to identify this, we must calculate the number of moles of Zn and CuSO4
- Moles = mass / molar mass
- Moles of Zn = 5.023 / 65.38
- Moles of Zn = 0.0768 moles
- Moles = concentration x volume (dm3)
- Moles of CuSO4 = 1.00 x 0.050
- Moles of CuSO4 = 0.050 moles
- Moles = mass / molar mass
- Therefore, the CuSO4 is the limiting reagent and should be used to calculate ΔH for the reaction
- Calculate the energy change, Q:
- Q = mcΔT
- Q = 50 x 4.18 x 37
- Q = 7733 J
- Q = mcΔT
- Calculate ΔH:
- ΔH = - Q / moles of CuSO4, using -Q as it is an exothermic reaction
- ΔH = - 7733 / 0.050
- ΔH = -154660 J mol-1
- ΔH = -154.6 kJ mol-1
- ΔH = - Q / moles of CuSO4, using -Q as it is an exothermic reaction
Worked Example
Calculate the energy change per mole for a reaction where 25 cm3 of 2.00 mol dm-3 hydrochloric acid was neutralised by 25 cm3 of 2.00 mol dm-3 sodium hydroxide. The temperature increased by 13.5 oC.
Answer:
Step 1: Write an equation for the reaction occurring
HCl + NaOH → NaCl + H2O
Step 2: Calculate the energy change for the amount of reactants in the reaction vessel (remember that the mass equals the mass of acid and alkali)
-
- Q = mcΔT
- Q = 50 x 4.18 x 13.5
- Q = 2821.5 J
- Q = mcΔT
Step 3: Calculate the number of moles of HCl (remember that neutralisation has occurred)
-
- Moles of HCl = concentration x volume (dm3)
- moles of HCl = 2 x 0.025
- moles of HCl = 0.05 moles
- Moles of HCl = concentration x volume (dm3)
Step 4: Calculate ΔH, using -Q as it is an exothermic reaction
-
- ΔH = - Q / moles of HCl
- ΔH = - 2821.5 / 0.05
- ΔH = -56430 J mol-1
- ΔH = -56.4 kJ mol-1
- ΔH = - Q / moles of HCl
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









