- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记15.1.4 Dissolution Energy Cycles
Dissolution Energy Cycles
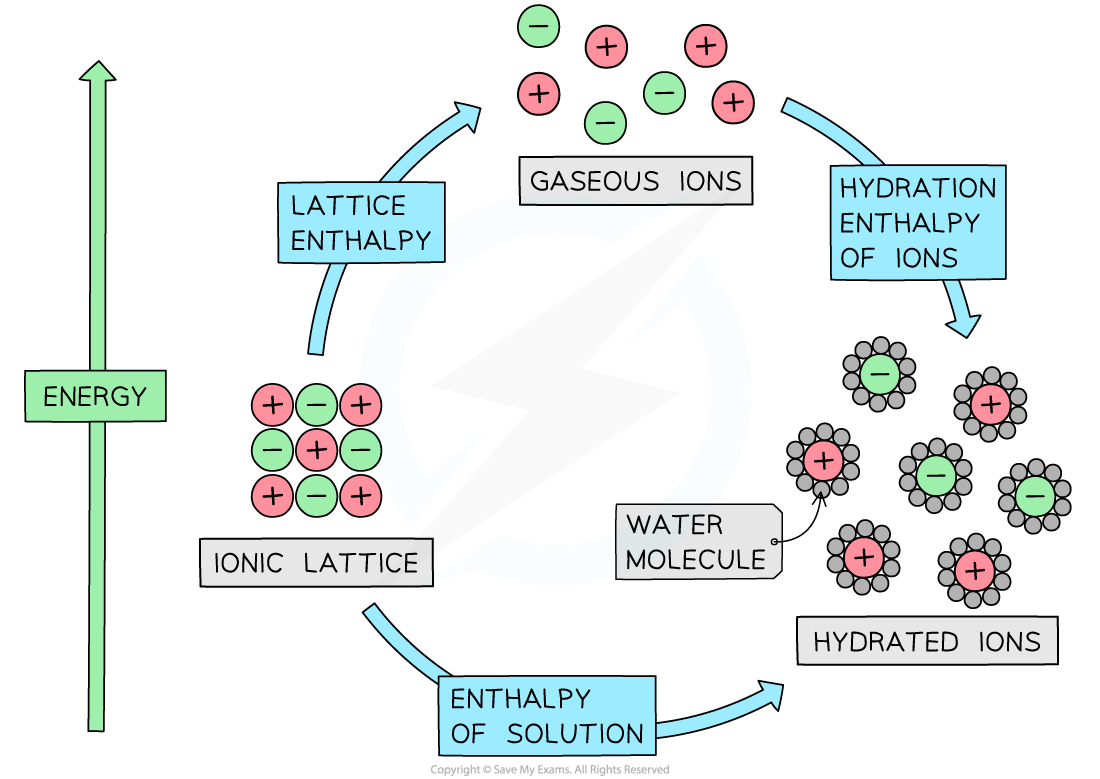
The relationship between lattice enthalpy, hydration enthalpies and enthalpy of solution
- From the diagram we can see that the relationship is
Enthalpy of solution = lattice enthalpy + hydration enthalpy
- The hydration enthalpy is the sum of the hydration enthalpies of each ion
- If there is more than one cation or anion, such as in MgCl2, then you must multiply by the appropriate coefficient for that ion
Calculations from Dissolution Cycles
- In order to calculate either ΔHsolꝋ , ΔHlatꝋ or ΔHhydꝋ from given data we must apply Hess's Law
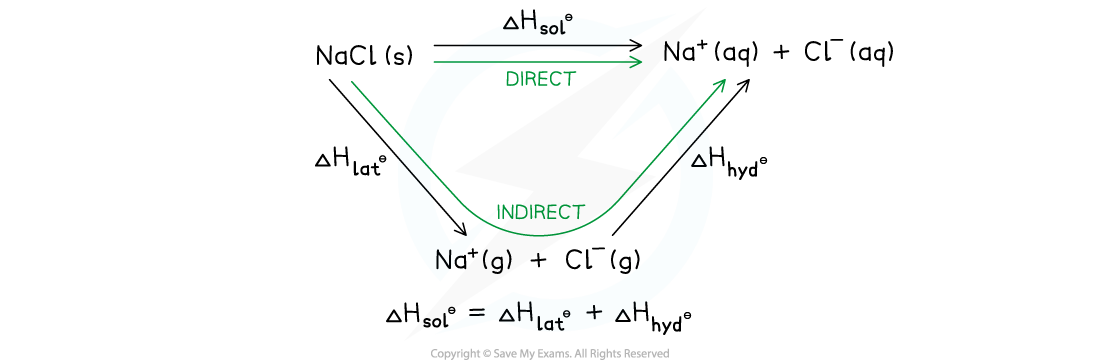
Energy cycle showing the application of Hess's Law to sodium chloride
- The energy cycle shows that there are two routes to go from the gaseous ions to the ions in an aqueous solution:
-
- Route 1: going from ionic solid → ions is the gaseous phase → ions in aqueous solution (this is the indirect route)
- Route 2: going from ionic solid → ions in aqueous solution (this is the direct route)
-
- According to Hess’s law, the enthalpy change for both routes is the same, such that:
ΔHsolꝋ = ΔHlatꝋ + ΔHhydꝋ
- Each ion will have its own enthalpy change of hydration, ΔHhydꝋ, which will need to be taken into account during calculations
- The hydration enthalpy is the sum of the hydration enthalpies of each ion
- The total ΔHhydꝋ is found by adding the ΔHhydꝋ values of both anions and cations together
- If there is more than one cation or anion, such as in MgCl2, then you must multiply by the appropriate coefficient for that ion
- This can also be represented as a Born-Haber cycle with the same direct and indirect route
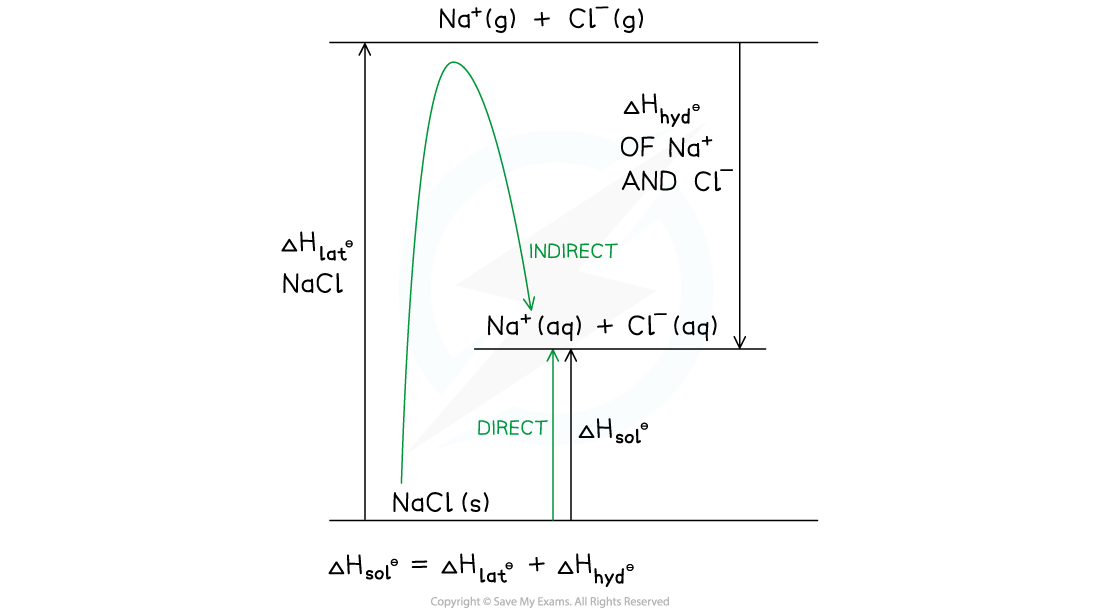
Born-Haber cycle for sodium chloride
Worked Example
Calculate the enthalpy change of solution ΔHsolꝋ of calcium fluoride, CaF2 using the following data:
- ΔHlatꝋ CaF2 = +2651 kJ mol-1
- ΔHhydꝋ Ca2+ = -1616 kJ mol-1
- ΔHhydꝋ F- = -504 kJ mol-1
Answer:
Option 1 - Drawn as a Hess's Law cycle:
CaF₂ Hess’s Law Cycle
It is possible to complete this question by drawing a Born-Haber cycle, but examiners see mistakes more often on hydration and solution enthalpy questions when they are completed using a Born-Haber cycle.
Option 2 - Drawn as a Born-Haber cycle:
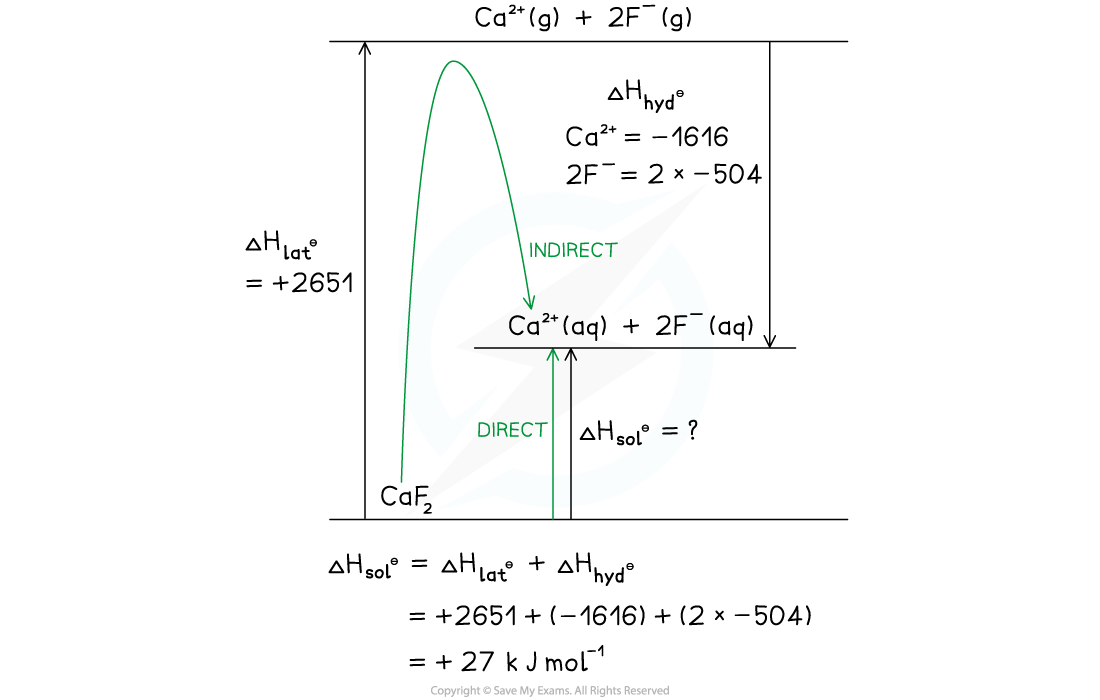
CaF₂ B-H cycle
Worked Example
Calculate the value of the enthalpy of hydration, ΔHhydꝋ, for the NH4+ ion using the following data:
- ΔHlatꝋ NH4Cl = +705 kJ mol-1
- ΔHsolꝋ NH4Cl = +14.78 kJ mol-1
- ΔHhydꝋ Cl- = -359 kJ mol-1
Answer:
Drawn as a Hess's Law cycle: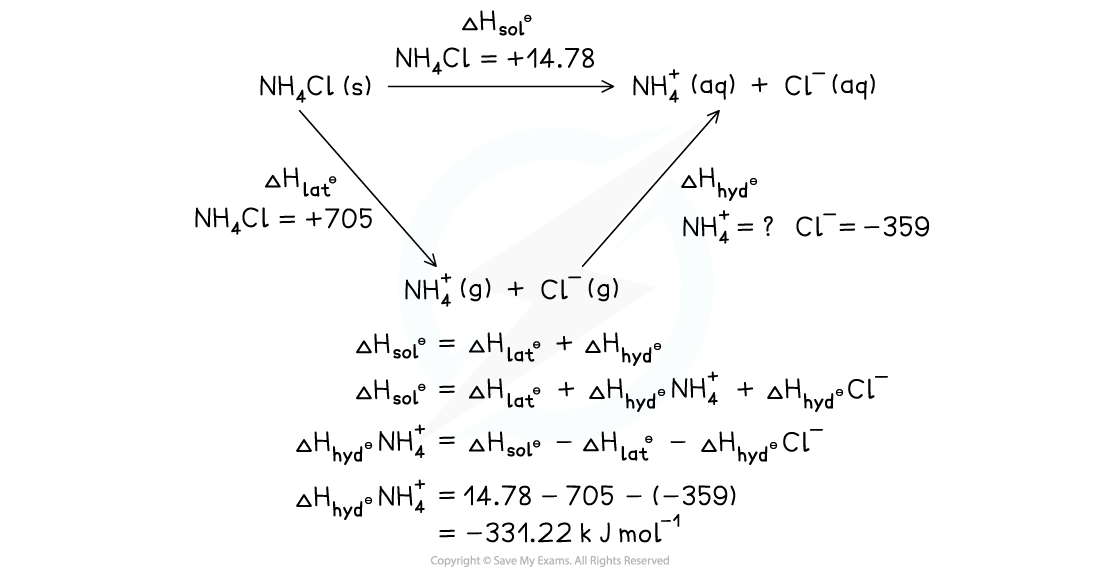
Exam Tip
Exam problems in this topic often show diagrams with missing labels which you have to complete and find unknown values.The key to success in energy cycle calculations is not to panic, but have a careful step-by-step approach, show your workings and use brackets to separate mathematical operations from the enthalpy changes.
Size & Charge of Ions & Hydration Enthalpy
- Hydration enthalpies are always negative values (exothermic)
- When an ionic solid dissolves in water, positive and negative ions are formed
- Water is a polar molecule with a δ- oxygen (O) atom and δ+ hydrogen (H) atoms which will form ion-dipole attractions with the ions present in the solution
- The oxygen atom in water will be attracted to the positive ions and the hydrogen atoms will be attracted to the negative ions
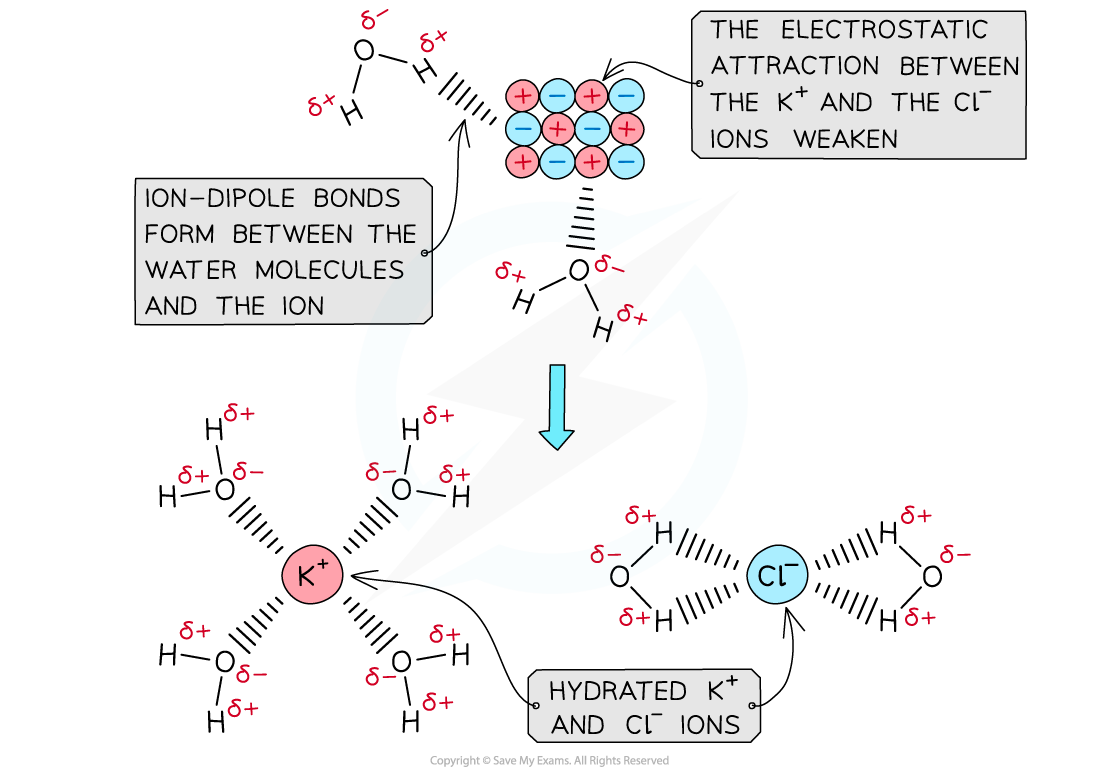
- The polar water molecules will form ion-dipole bonds with the ions in solution causing the ions to become hydrated
- The size of the hydration enthalpy is governed by the amount of attraction between the ions and the water molecules
- The smaller the ion, the stronger the attraction between the ions and the water molecules
- As you go down a group, the ionic radius increases so attraction decreases and the the hydration enthalpy will become less exothermic
- Overall, a smaller ion gives a more exothermic hydration enthalpy
- The more highly charged the ion; the stronger the attraction
- The hydration enthalpies of 2+ ions in group 2 are much more exothermic than those of 1+ ions in group 1 as the attraction between the 2+ ions and the water molecules is stronger
- Overall, a greater charge on the ion gives a more exothermic hydration enthalpy
Hydration enthalpies of group 1 and group 2 ions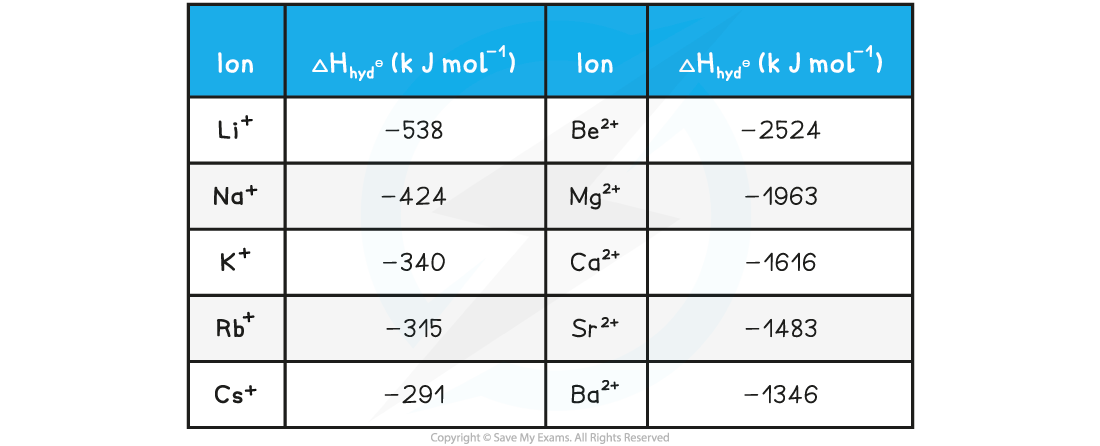
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









