- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记15.1.1 Key Enthalpy Terms
Key Enthalpy Terms
Ionisation energy
- The ionisation energy (ΔHIEꝋ) is the standard enthalpy change that occurs on the removal of 1 mole of electrons from 1 mole of gaseous atoms or positively charged ions
- Ionisation energy is always endothermic as energy is need to overcome the attraction between an electron and the nucleus
- The first ionisation energy (ΔHIE1ꝋ) is the energy required to remove one mole of electrons from 1 mole of gaseous atoms of an element to form 1 mole of 1+ ions in the gaseous phase
ΔHIE1ꝋAl (g) → Al+ (g) + e– ΔHIE1ꝋ = +577 kJ mol-1
- The second ionisation energy (ΔHIE2ꝋ) is the energy required to remove 1 mole of electrons from 1 mole of gaseous 1+ ions to form 1 mole of 2+ ions in the gaseous phase
ΔHIE2ꝋAl+ (g) → Al2+ (g) + e– ΔHIE2ꝋ = +1820 kJ mol-1
Enthalpy of Atomisation
- The enthalpy of atomisation (ΔHatꝋ) is the standard enthalpy change that occurs on the formation of 1 mole of separate gaseous atoms an element in its standard state
- The ΔHatꝋ is always endothermic as energy is always required to break any bonds between the atoms in the element or to break the element into its gaseous atoms
- Since this is always an endothermic process, the enthalpy change will always have a positive value
Na (s) → Na (g) ΔHatꝋ = +108 kJ mol -1
½Cl (g) → Cl (g) ΔHatꝋ = +122 kJ mol -1
Electron Affinity
- The electron affinity (ΔHEAꝋ) of an element is the energy change when 1 mole of electrons is gained by 1 mole of gaseous atoms of an element to form 1 mole of gaseous ions under standard conditions
- For example, the first electron affinity of chlorine is:
Cl (g)+ e– → Cl– (g) ΔHEAꝋ = -364 kJ mol-1
- The first electron affinity is always exothermic as energy is released when electrons are attracted to the atoms
- However, the second electron affinity of an element can be endothermic as illustrated by oxygen:
O– (g) + e– → O2- (g) ΔHEAꝋ = +844 kJ mol-1
- This is because a large force of repulsion must be overcome between the negatively charged ion and the second electron requiring a large input of energy
Lattice Enthalpy
- The lattice enthalpy (ΔHlatꝋ) is defined as the standard enthalpy change that occurs on the formation of 1 mole of gaseous ions from the solid lattice
- The ΔHlatꝋ is always endothermic as energy is always required to break any bonds between the ions in the lattice
- Since this is always an endothermic process, the enthalpy change will always have a positive value
NaCl (s) → Na+ (g) + Cl- (g) ΔHlatꝋ = +790 kJ mol -1
Enthalpy of Solution
- The standard enthalpy change of solution (ΔHsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution
- The symbol (aq) is used to show that the solid is dissolved in sufficient water
- ΔHsolꝋ can be exothermic (negative) or endothermic (positive)
LiBr(s) → LiBr(aq) ΔHsolꝋ = -48.8 kJ mol -1
KCl (s) → KCI (aq) ΔHsolꝋ = +17.2 kJ mol -1
CaCl2(s) → CaCl2 (aq) ΔHsolꝋ = -82.8 kJ mol -1
Enthalpy of Hydration
- The standard enthalpy change of hydration (ΔHhydꝋ) is the enthalpy change when 1 mole of a specified gaseous ion dissolves in sufficient water to form an infinitely dilute solution
Mg2+ (g) → Mg2+ (aq) ΔHhydꝋ = -1963 kJ mol -1
Br- (g) → Br- (aq) ΔHhydꝋ = -328 kJ mol -1
- Hydration enthalpies are the measure of the energy that is released when there is an attraction formed between the ions and water molecules
- Hydration enthalpies are exothermic
- The term solvation is used in place of hydration if water has been replaced by another solvent
- When an ionic solid dissolves in water, positive and negative ions are formed
- Water is a polar molecule with a δ- oxygen (O) atom and δ+ hydrogen (H) atoms which will form ion-dipole attractions with the ions present in the solution
- The oxygen atom in water will be attracted to the positive ions and the hydrogen atoms will be attracted to the negative ions
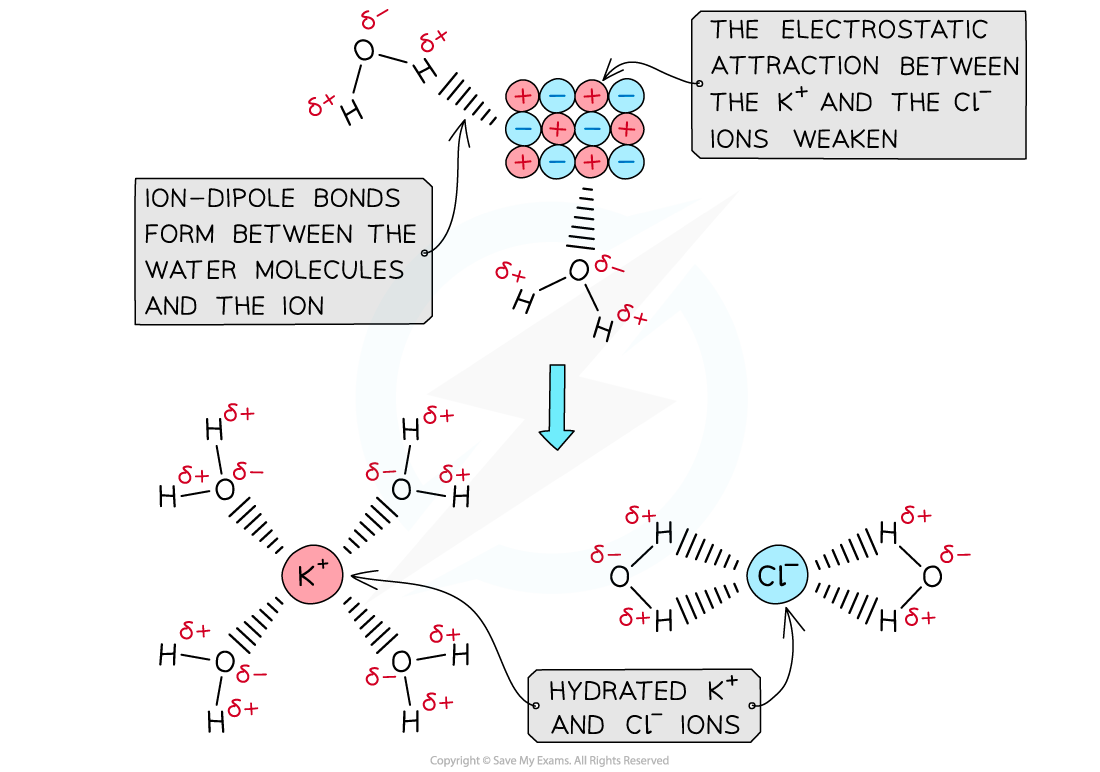 The polar water molecules will form ion-dipole bonds with the ions in solution causing the ions to become hydrated
The polar water molecules will form ion-dipole bonds with the ions in solution causing the ions to become hydrated
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








