- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记14.1.4 Formal Charge
Formal Charge
- A limitation of the model of covalent bonding is that when drawing Lewis structures for molecules, it is sometimes possible to come up with more than one structure while still obeying the octet rule
- This leads to the problem of deciding which structure is appropriate and is consistent with other information such as spectroscopic data on bond lengths and electron density
- One approach to determining which is the preferred structure is to determine the formal charge (FC) of all the atoms present in the molecule
- It is a kind of electronic book keeping involving the bonding, non-bonding and valence electrons
- Formal charge is described as the charge assigned to an atom in a molecule, assuming that all the electrons in the bonds are shared equally between atoms, regardless of differences in electronegativity
- The formula for calculating FC is
FC= (number of valence electrons) - ½(number of bonding electrons) - (number of non-bonding electrons)
or
FC= V - ½B - N
- The Lewis structure which is preferred is the one which:
- the difference in FC of the atoms is closest to zero
- has negative charges located on the most electronegative atoms
- The process of drawing a Lewis structure has been covered previously, but here is a reminder of how to draw the Lewis structure of tetrachloromethane, CCl4
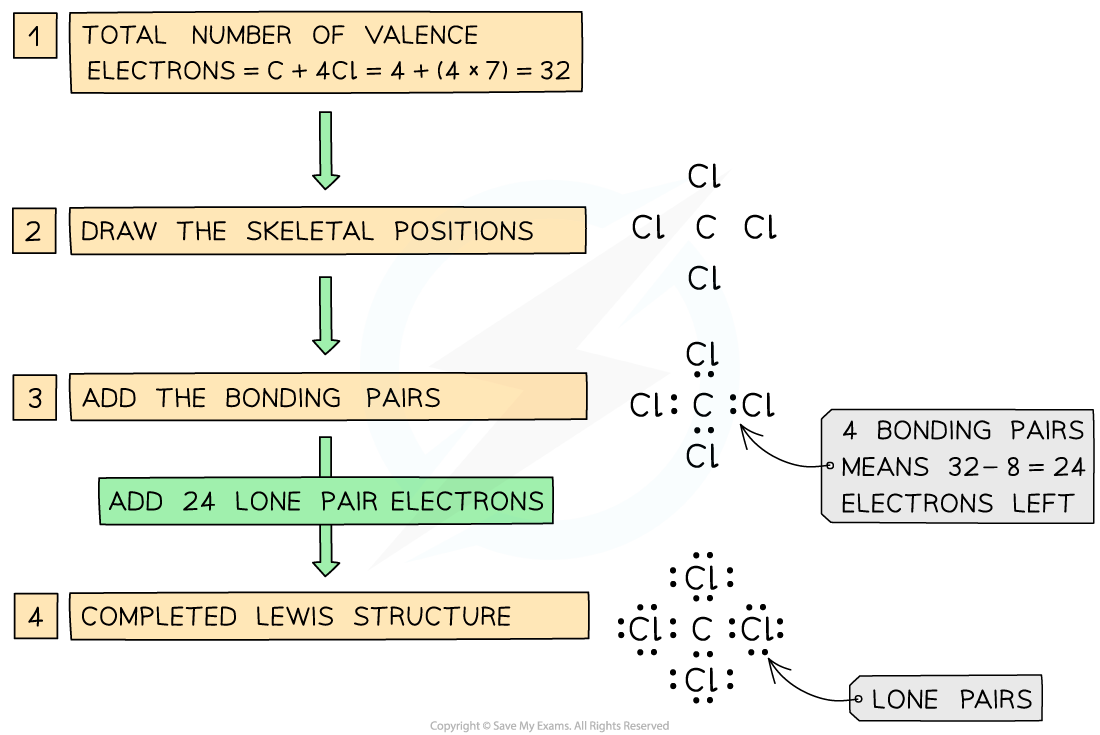
Steps in drawing the Lewis structure for CCl4
- To work our the formal charge of the C and Cl atoms in the structure simply apply the FC formula:
FC for carbon = (4) - ½(8) - 0 = 0
FC for chlorine = (7) - ½(2) - 6 = 0
- Notice that formal charge is calculated for one of each type of atom and does not count the total number of atoms in the molecule
Worked Example
What is the formal charge on boron in the BH4- ion?
Answer
-
- Boron is a group 13 element, so has 3 valence electrons. Hydrogen has one valence electron and the charge on the ion is -1, so there are 8 electrons in the diagram. The Lewis structure is therefore:

Lewis structure of BH4-
-
- The number of bonded electrons is 8 and the number of non-bonded electrons is zero. So the formal charge on B is:
FC (B) = (3) - ½(8) - 0 = -1
Applying Formal Charge
- It is possible to draw three resonance structures for sulfur dioxide, SO2:

The three resonance structures of sulfur dioxide
- The first structure is an illustration of the expansion of the octet as the sulfur has 10 electrons around it
- Formal charge can be used to decide which of the Lewis structures is preferred
- The FC on the first structure is as follows:
FC on sulfur = (6) - ½(8) -(2) = 0
FC on oxygen = (6) - ½(4) -(4) = 0
Difference in FC = ΔFC = FCmax- FCmin = 0
- The FC on the second (and third) structures is as follows:
FC on sulfur = (6) - ½(6) -(2) = +1
FC on left side oxygen = (6) - ½(2) -(6) = -1
FC on right side oxygen = (6) - ½(4) -(4) = 0
Difference in FC = ΔFC = FCmax- FCmin = 2
Worked Example
What is the formal charge on the two resonance structures shown?
Resonance structures of carbon dioxide
Deduce which is the preferred structure.
Answer
Structure I
FC on carbon = (4) - ½(8) -(0) = 0
FC on oxygen = (6) - ½(4) -(4) = 0
Difference in FC = ΔFC = FCmax- FCmin = 0
Structure II
FC on carbon = (4) - ½(8) -(0) = 0
FC on left oxygen = (6) - ½(6) -(2) = +1
FC on right oxygen = (6) - ½(2) -(6) = -1
Difference in FC = ΔFC = FCmax- FCmin = 2
Structure I is the preferred structure as the difference is zero
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









