- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记13.1.5 Colour in Transition Metals
Crystal Field Theory
Perception of colour
- Most transition metal compounds appear coloured. This is because they absorb energy corresponding to certain parts of the visible electromagnetic spectrum
- The colour that is seen is made up of the parts of the visible spectrum that aren’t absorbed
- For example, a green compound will absorb all frequencies of the spectrum apart from green light, which is transmitted
- The colours absorbed are complementary to the colour observed
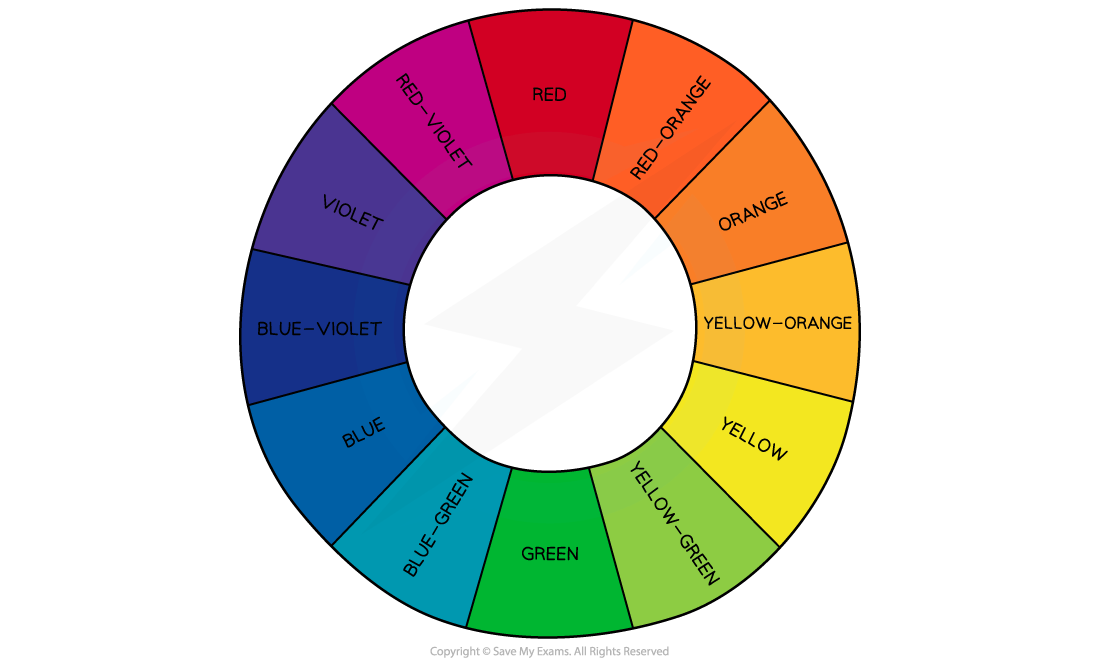
The colour wheel showing complementary colours in the visible light region of the electromagnetic spectrum
- Complementary colours are any two colours which are directly opposite each other in the colour wheel
- For example, the complementary colour of red is green and the complementary colours of red-violet are yellow-green
Crystal Field Theory (CFT)
- The crystal field theory is a model based on electrostatic point charges and is used to explain colour in transition metal compounds
- In a transition metal atom, the five orbitals that make up the d-subshell all have the same energy. The term for this is degenerate
- However, when ligands are attached to a transition metal ion, the electric field formed by the lone pairs of electrons on the ligands repel the electrons in the d-subshell causing the d-orbitals to split in energy
- The dative bonding from the ligands causes the five d orbitals to split into two sets
- These two sets are not equal in energy and are described as being non-degenerate orbitals
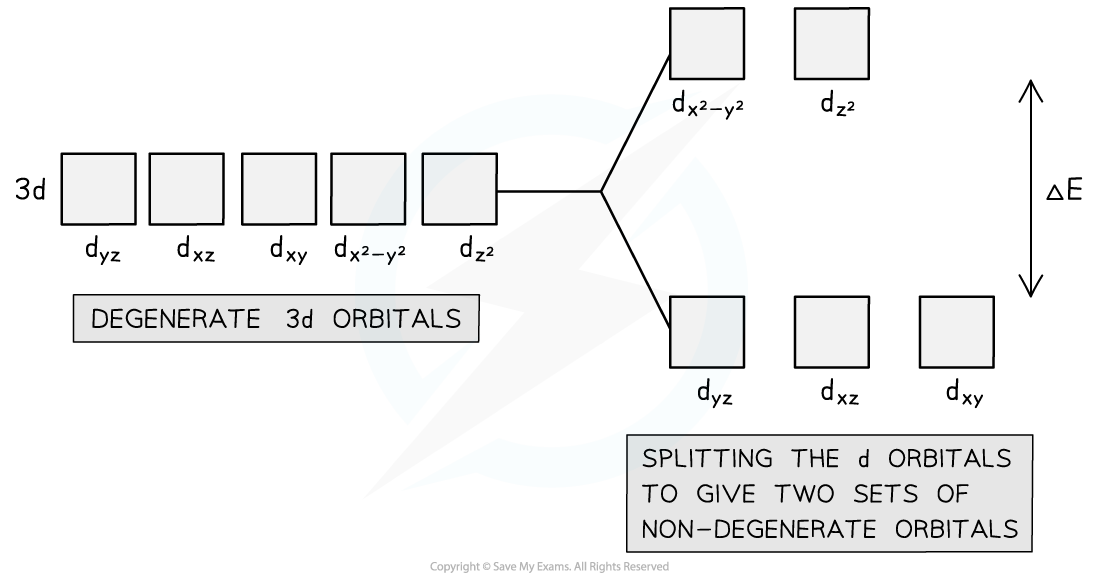
Upon bonding to ligands, the d orbitals of the transition element ion split into two non-degenerate sets of orbitals
Splitting in octahedral complexes
- In octahedral complexes, there are six ligands arranged around the central metal ion
- The lone pairs of the ligands repel the electrons in the x2-y2 and z2 orbitals of the metal ion more than they repel the electrons in the 3dyz, 3dxz, and 3dxy orbitals
- This is because the 3dx2-y2 and 3dz2 orbitals line up with the dative bonds in the complex’s octahedral shape
- The incoming ligands are attached to or approaching the central metal ion along the x, y and z axes, and the 3dx2-y2 and 3dz2 orbitals have lobes along these axes
- The electrons in these two orbitals are closer to the bonding electrons, so there is more repulsion
- This means that when the d orbitals split, the 3dx2-y2 and 3dz2 orbitals are at a slightly higher energy level than the other three
- The difference in energy between the non-degenerate d orbitals is labelled as ΔE
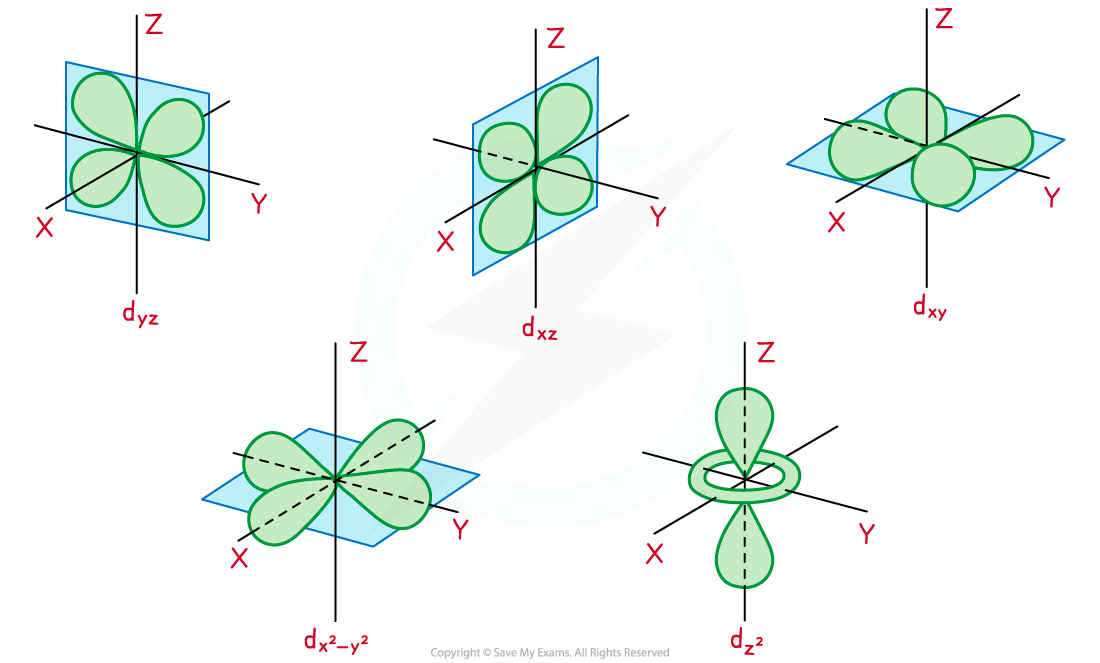
Diagram showing the shapes and orientation of the five d-orbitals
Absorption of light
- When white light passes through a solution of aqueous nickel(II) sulfate an electron in the lower energy d-orbitals is excited and jumps up into the higher energy d-orbitals
- A photon of red light is absorbed and light of the complementary colour (green) is transmitted
- This is why nickel(II) sulfate solution appears green
- The energy of the separation is ΔE corresponding to a wavelength of about 630-700 nm
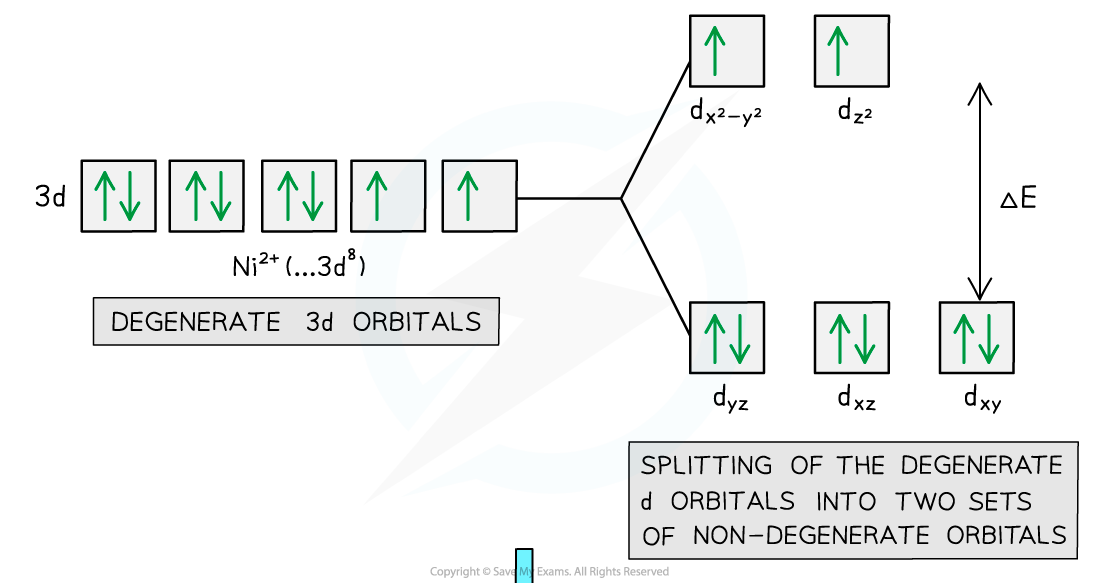
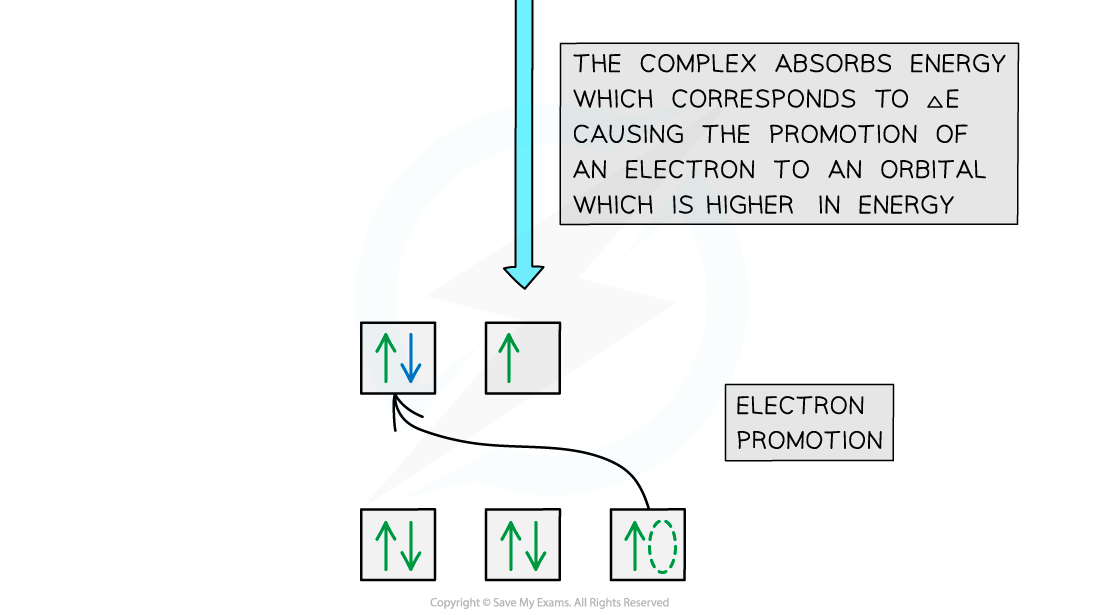
Electron promotion in a Ni(II) complex when light shines on the solution
Exam Tip
The colour wheel is given in Section 17 of the Data booklet, so there is no need to learn it. There are different splitting patterns possible but you are only required to know the octahedral splitting pattern discussed above.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









