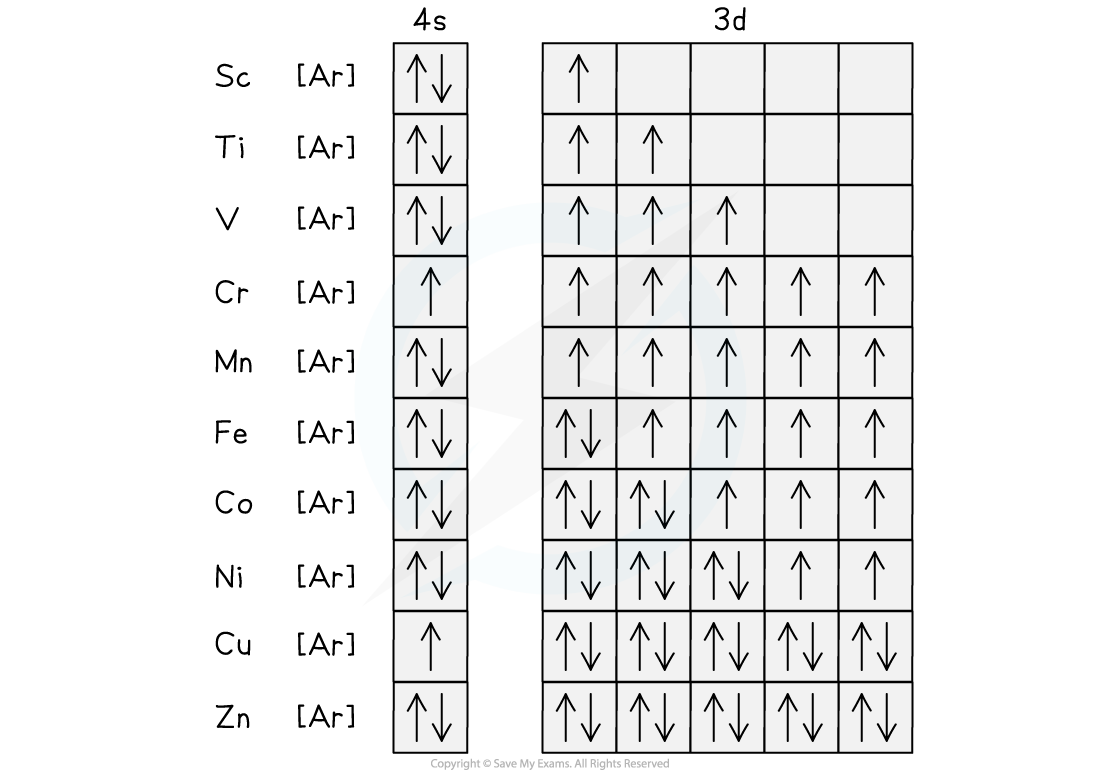
The electrons in titanium are arranged in their orbitals as shown. The unpaired electrons can be temporarily aligned in an external field.
- Most of the transition metals and their ions are paramagnetic as they have unpaired electrons
- Paramagnetism increases with the number of unpaired electrons, so it generally increases across the d-block up to a maximum with chromium and then decreases
- Zinc has no unpaired electrons so is not paramagnetic

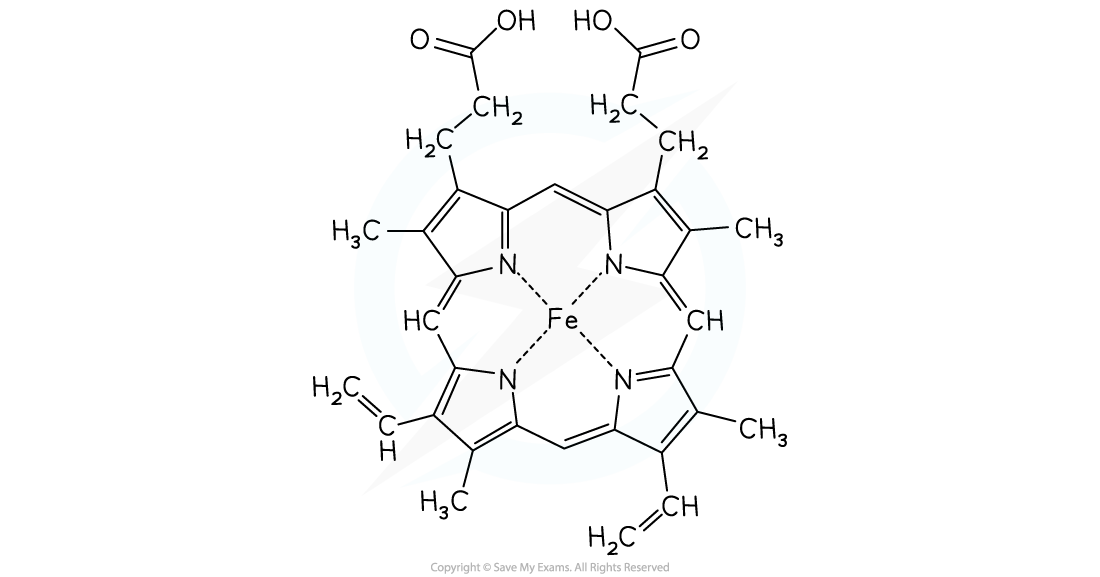
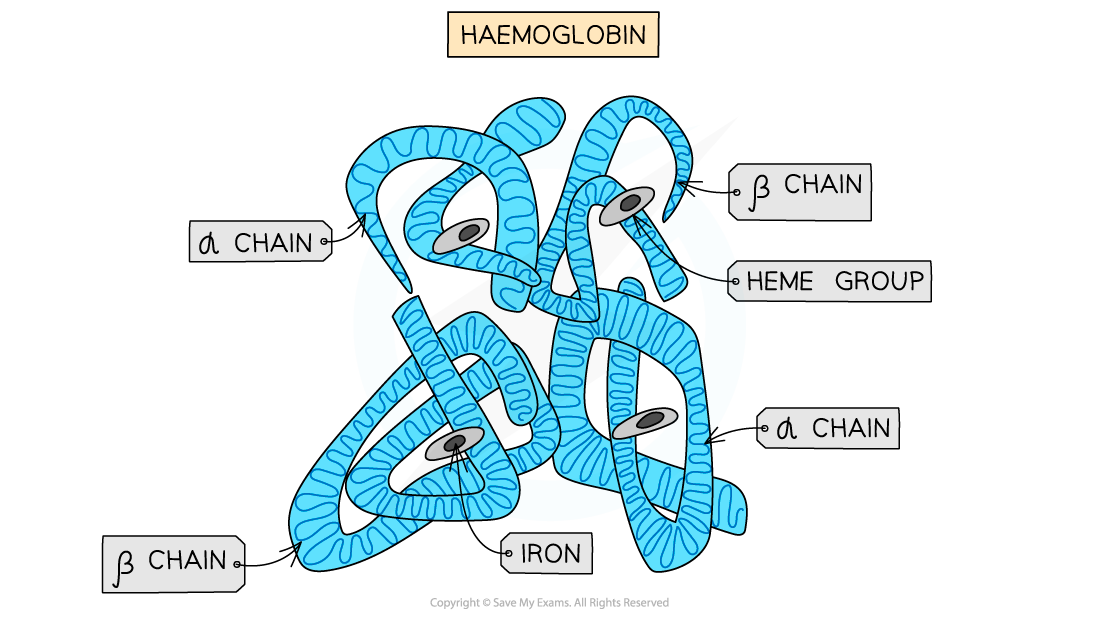 The structure of haemoglobin
The structure of haemoglobin