- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记12.1.1 Emission Spectra & First Ionisation Energy
Convergence Limits on an Emission Spectrum
Emission Spectra
- Electrons move rapidly around the nucleus in energy shells
- Heat or electricity can be used to excite an electron to a higher main energy level
- These range from n = 1 (ground state) to n = ∞
- When the electrons 'fall' back down they must lose the energy difference between the two energy levels. This loss of energy is performed by releasing electromagnetic energy in the form of infrared, visible light or ultraviolet radiation.
- When the electron falls back to n = 1 (ground state) the energy released is in the ultraviolet region of the spectrum
- This corresponds to the Lyman series
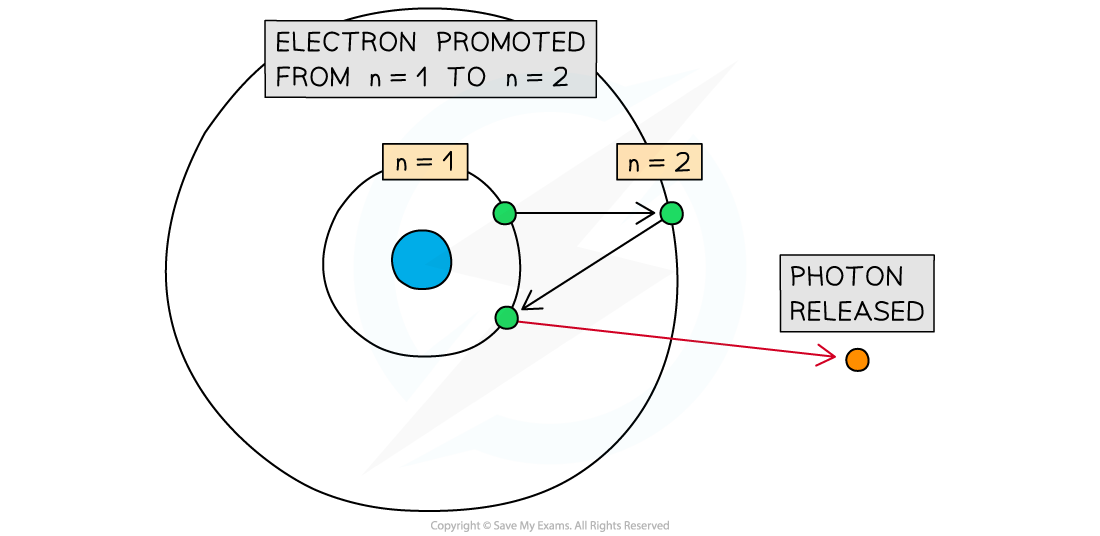
Promotion of an electron from the ground state (n=1) to n=2
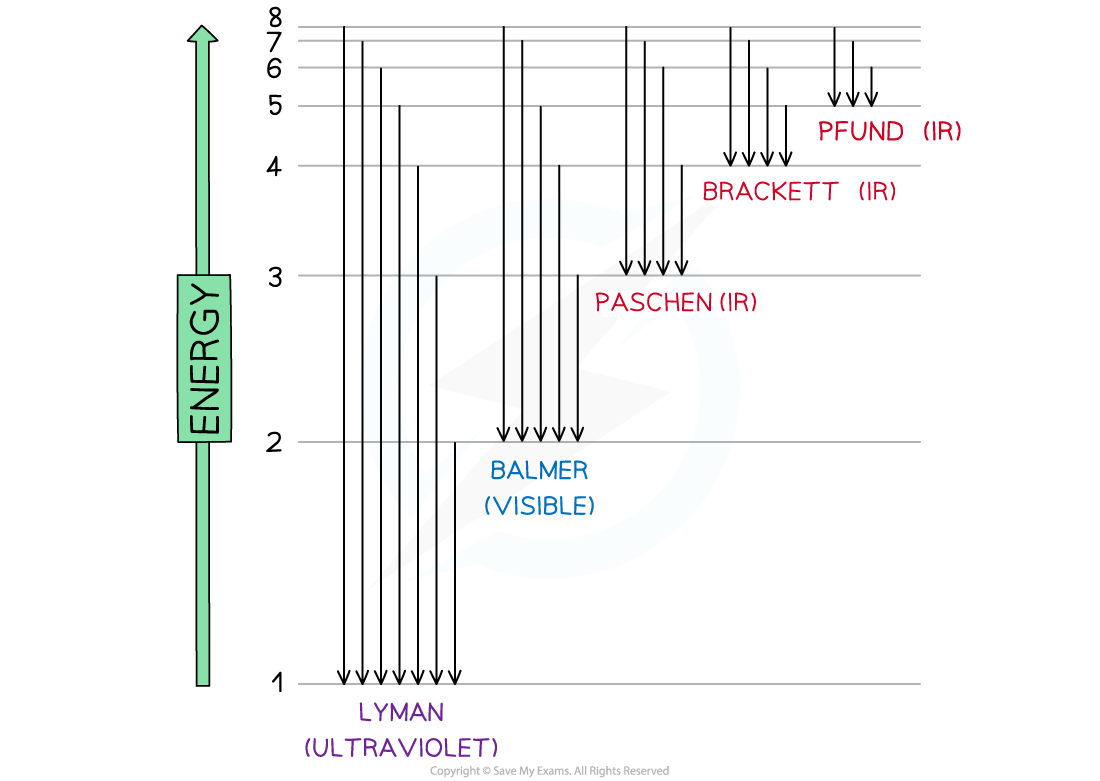
Electron jumps in the hydrogen spectrum
- This gives evidence for Bohr's model which is the idea that electrons exist in discrete energy levels so an exact amount of energy is required for an electron to 'jump' an energy level, a little like a ladder
- There are however limitations to this model
- Assumes positions of electrons are fixed
- Assumes energy levels are spherical in nature
- Bohr limited calculations to hydrogen only, so does not explain the line spectra of other elements containing more than one electron
The Limit of Convergence
- As the line spectra is produced the lines will become closer together
- Where the lines appear to meet is called the limit of convergence
- The convergence limit is the frequency at which the spectral lines converge
- The energy required for an electron to escape the atom, or reach the upper limit of convergence, is the ionisation energy
- The frequency of the radiation in the emission spectrum at the limit of convergence can be used to determine the first ionisation energy or IE1
- In the Lyman series for the hydrogen atom (UV region), the frequency at the limit of convergence relates to the energy given out when an electron falls from n = ∞ to n = 1
- For hydrogen, the lines converge to a limit with a wavelength of 91.16 nm or 91.16 × 10−9 m
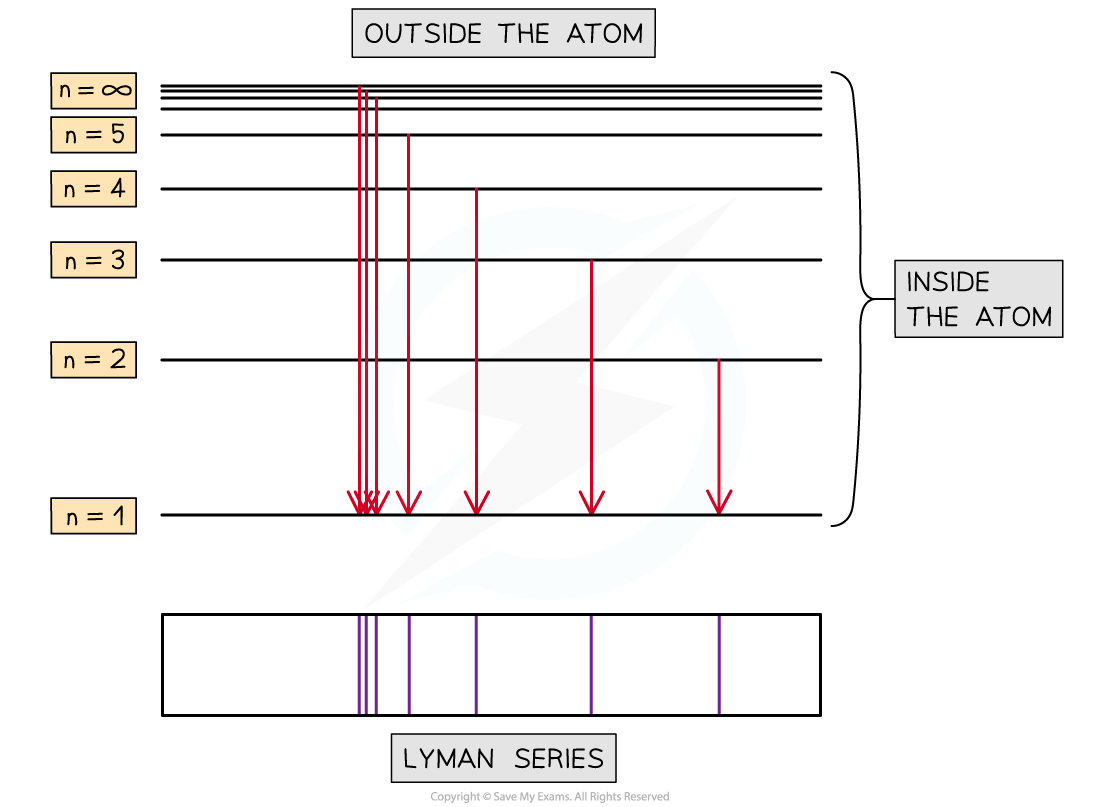
Lyman series (ultra-violet radiation) corresponds to transitions between higher shells and the ground state (n=1)
Calculating First Ionisation Energy
Calculating First Ionisation Energy
- When dealing with the Lyman series the largest transitions represent the fall from the infinite level to n=1
- In reverse, it can be considered to be equal to the ionisation energy (note that ionisation energy is given per mole of atoms)
- Therefore, the first ionisation energy (IE1) of an atom can be calculated using the frequency (or wavelength) of the convergence limit
- We can do this by using the following equations
ΔE = h ν
c = ν λ
- In order to calculate first ionisation energy (IE1) we must first calculate the frequency using the given data and rearranging:
c = ν λ
as
ν = c ÷ λ
- Once we know the frequency, we can use this to calculate the ionisation energy

Worked Example
The convergence limit for the sodium atom has a frequency of 1.24 × 1015 s−1. Calculate the first ionisation energy of sodium in kJ mol−1.
Answer:
Step 1: Write out the equation to calculate the first ionisation energy (IE1)
ΔE = h ν
Step 2: Substitute in numbers from question and data booklet to give energy change per atom
IE1 = 6.63 × 10−34 × 1.24 × 1015
IE1 = 8.22 × 10−19 J atom−1
Step 3: Calculate the first ionisation energy per mole by multiplying by Avogadro's constant
IE1 = 8.22 × 10−19 × 6.02 × 1023
IE1 = 494 916 J mol−1
Step 4: Convert J mol−1 to kJ mol−1 by dividing by 1000
IE1 = 495 kJ mol−1
So the first ionisation energy (IE1) of sodium has been calculated as 495 kJ mol−1
Worked Example
The convergence limit for the hydrogen atom has a wavelength of 91.16 nm. Calculate the ionisation energy for hydrogen in kJ mol−1.
Answer:
Step 1: Calculate the frequency of the convergence limit, converting wavelength into m (nm to m = × 10−9)
c = ν λ
ν = c ÷ λ
ν = 3.00 × 108 ÷ 91.16 × 10−9
ν = 3.29 × 1015 s−1
Step 2: Substitute into the equation to calculate IE1 for one atom of hydrogen in J mol−1
ΔE = h ν
IE1 = 6.63 × 10−34 × 3.29 × 1015
IE1 = 2.18 × 10-18 J atom−1
Step 3: Calculate IE1 for 1 mole of hydrogen atoms
IE1 = 2.18 × 10−18 × 6.02 × 1023
IE1 = 1 313 491 J mol−1
Step 4: Convert J mol−1 to kJ mol−1
IE1 = 1313 kJ mol−1
So the first ionisation energy (IE1) of hydrogen has been calculated as 1313 kJ mol−1
Exam Tip
These equations are found in the data booklet so you don't need to learn them.Also, be careful to calculate the first ionisation energy (IE1) per mole by using Avogadro's constant (NA) 6.02 × 1023 and converting units to kJ mol−1.Finally, when working through calculations, keep the numbers in your calculator to avoid rounding up too early.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









