- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记11.2.4 Percentage Error
Percentage Error
- Percentage error is used to express the difference between a final calculated answer and an accepted or literature value
- It is calculated using the following formula
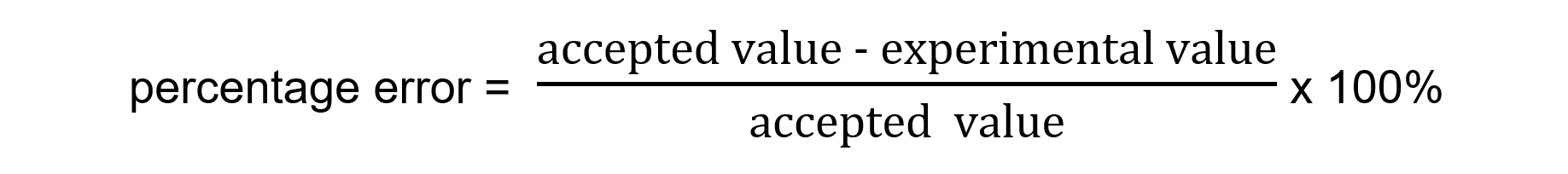
- You should be able to comment on any differences between the experimental and literature values
Worked Example
1.023 g of propan-1-ol (M = 60.11 g mol-1) was burned in a spirit burner and used to heat 200 g of water in a copper calorimeter. The temperature of the water rose by 30 oC.
- Calculate the enthalpy of combustion of propan-1-ol using this data.
- The literature value for this enthalpy change is -2021 kJ mol-1. Calculate the percentage error and comment on your findings
Answer 1:
Step 1: Calculate q
q = m x c x ΔT
q = 200 g x 4.18 J g-1 K-1 x 30 K = – 25 080 J
Step 2: Calculate the amount of propan-1-ol burned
moles = mass ÷ molar mass = 1.023 g ÷ 60.11 g mol-1 = 0.01702 mol
Step 3: Calculate ΔH
ΔH = q ÷ n = -25 080 J ÷ 0.01702 mol = – 1 473 560 J = -1 474 kJ = -1.5 x 103 kJ
Answer 2:
Using the formula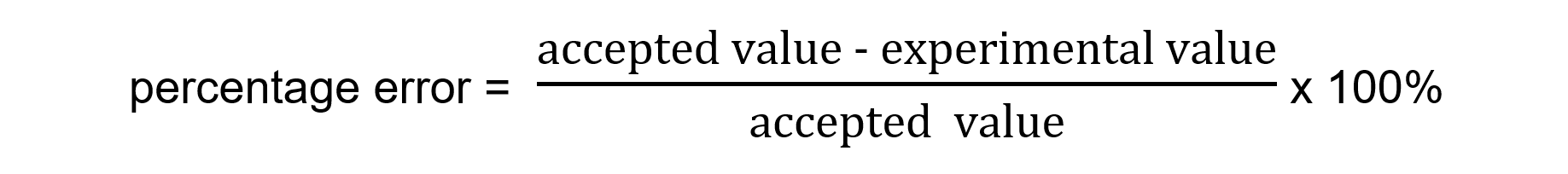
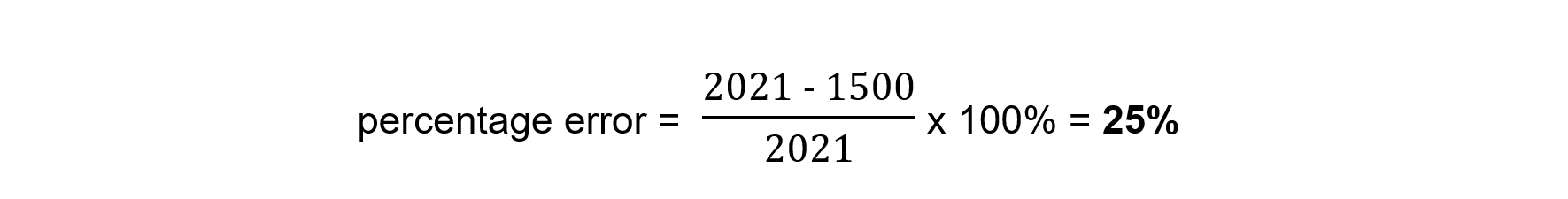 Heat losses are likely to be the largest source of error in this experiment
Heat losses are likely to be the largest source of error in this experiment
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









