- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记10.2.8 Addition Polymers
Polymers
Addition polymerisation
- Addition polymerisation is one of the most important addition reactions of alkenes which form the basis of the plastic industry
- Addition polymerisation is the reaction in which many monomers containing at least one C=C double bond form long chains of polymers as the only product
- Just like in other addition reactions of alkenes, the π-bond in each C=C bond breaks and then the monomers link together to form new C-C single bonds
- A polymer is a long-chain molecule that is made up of many repeating units
- The small, reactive molecules that react together to form the polymer are called monomers
- A polymerisation reaction can be represented by a general formula or by using displayed formulae
- E.g. poly(ethene) and poly(chloroethene) (also known as PVC) are polymers made up of the ethene and chloroethene monomers respectively and are commonly used in making plastics
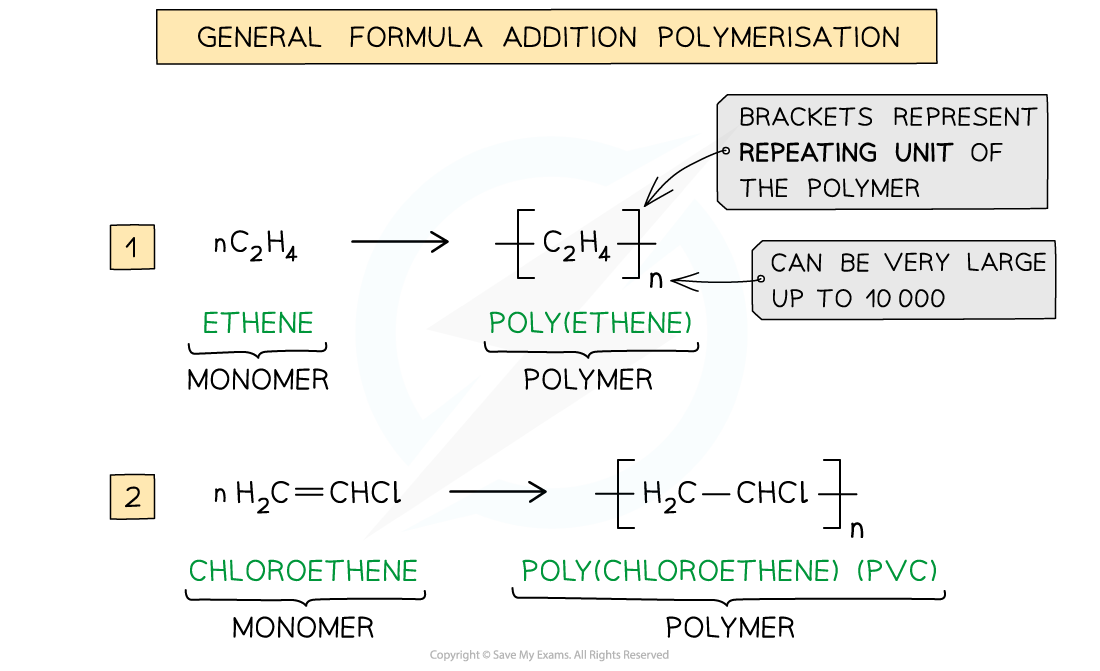
The general formulae of the addition polymerisation of ethene (1) and chloroethene (2)
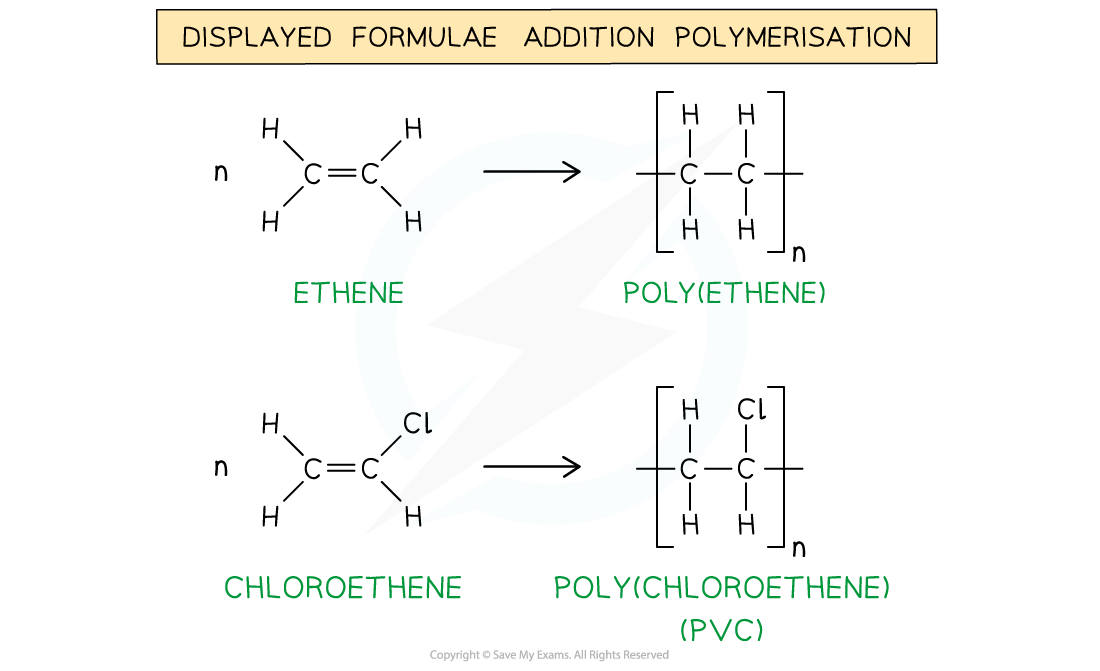
The addition polymerisation of ethene (1) and chloroethene (2)
- Just like any other addition reaction of alkenes, addition polymerisation gives only one product
Deducing repeat units
- A repeat unit is the smallest group of atoms that when connected one after the other make up the polymer chain
- It is represented by square brackets in the displayed and general formula
- In poly(alkenes) (such as poly(ethene)) and substituted poly(alkenes) (such as PVC) made of one type of monomer the repeating unit is the same as the monomer except that the C=C double bond is changed to a C-C single bond
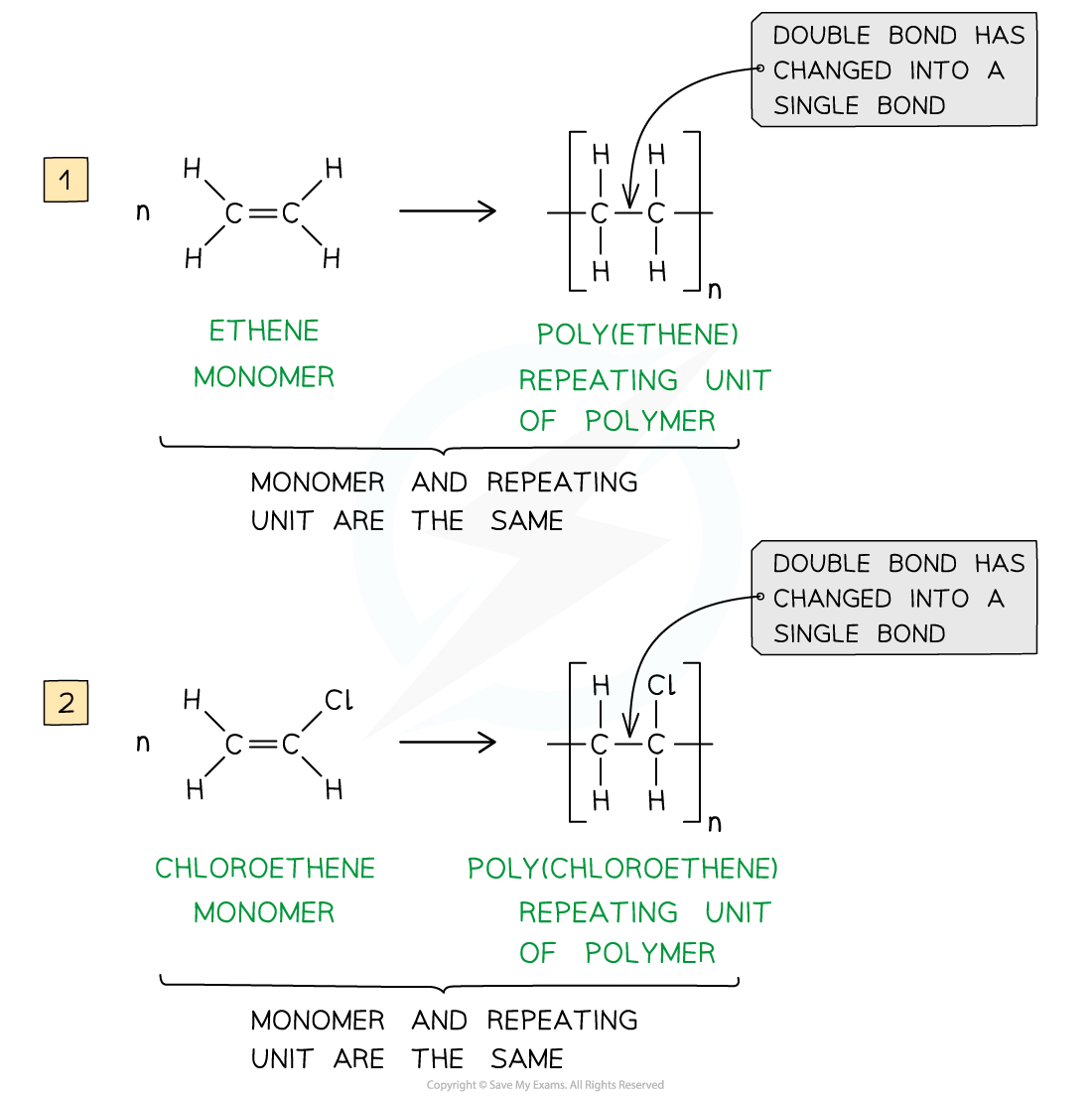
The repeating units of poly(ethene) and poly(chloroethene) are similar to their monomer except that the C=C bond has changed into a C-C bond
Worked Example
Identify the monomers present in the given sections of addition polymer molecules: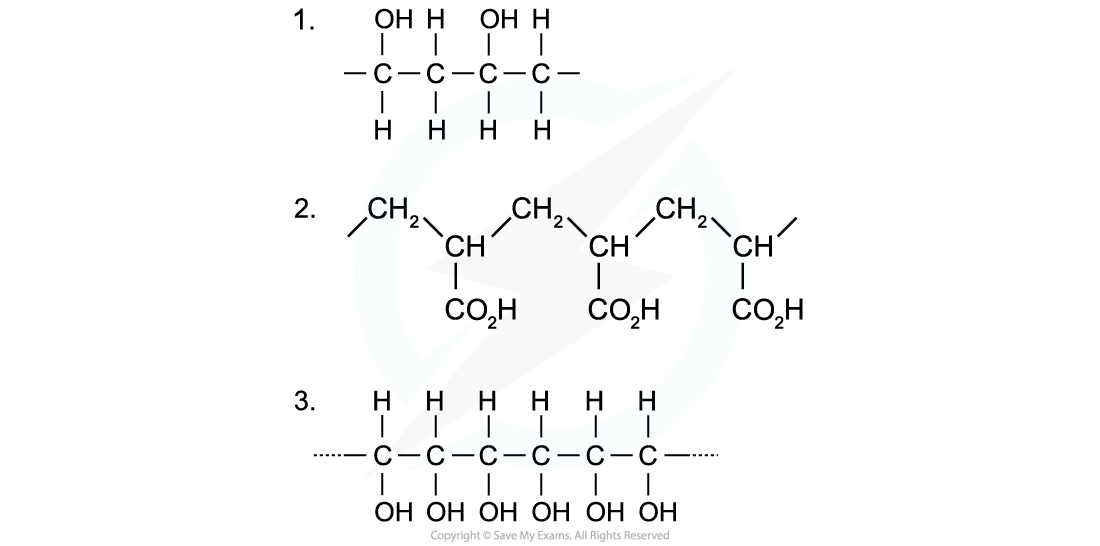
Answers:
Answer 1:
When ethenol (CH(OH)=CH2) is polymerised, the C-C double bond opens to produce a repeating unit of CH(OH)-CH2. This gives the polymer poly(ethenol)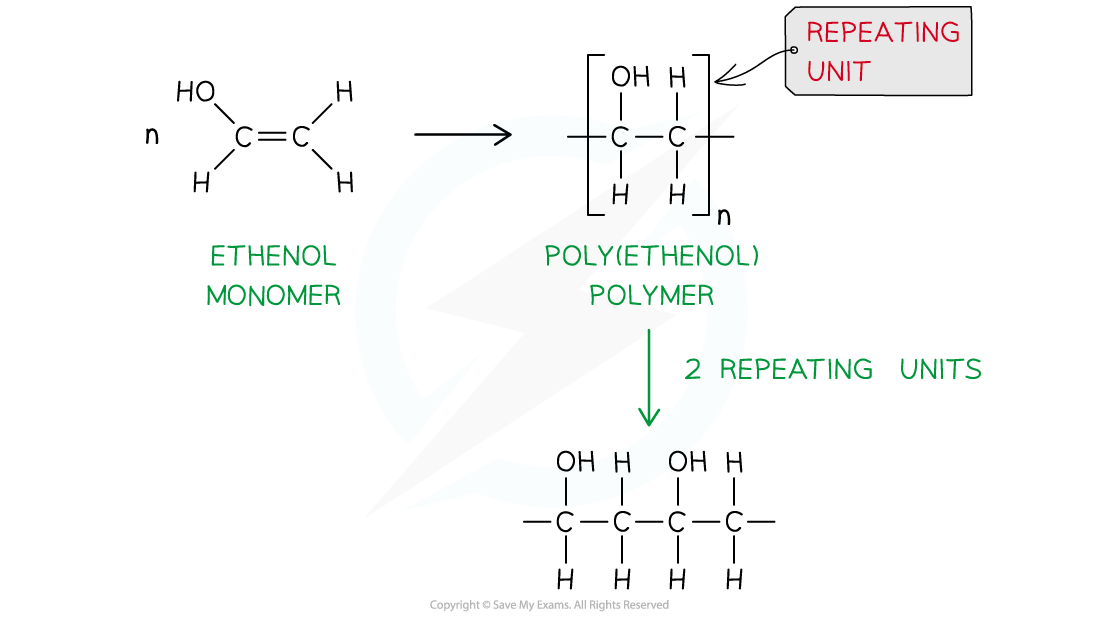
Answer 2:
-
- To find the monomer, first the repeating unit should be deduced. Repeating units have only 2 carbons in the polymer main chain
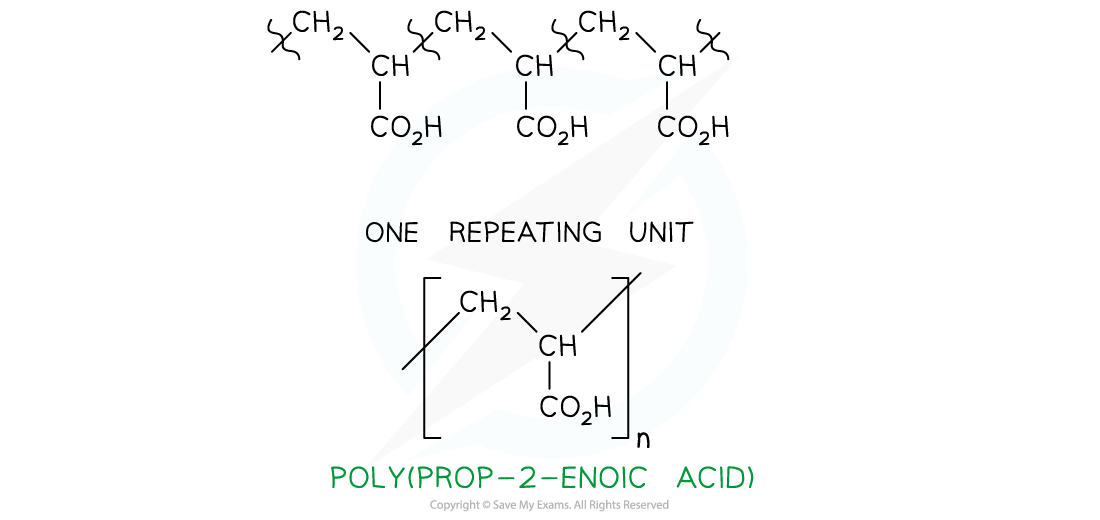
-
- Since the repeating unit is now found, it can be concluded that the monomer is prop-2-enoic acid

Answer 3:
-
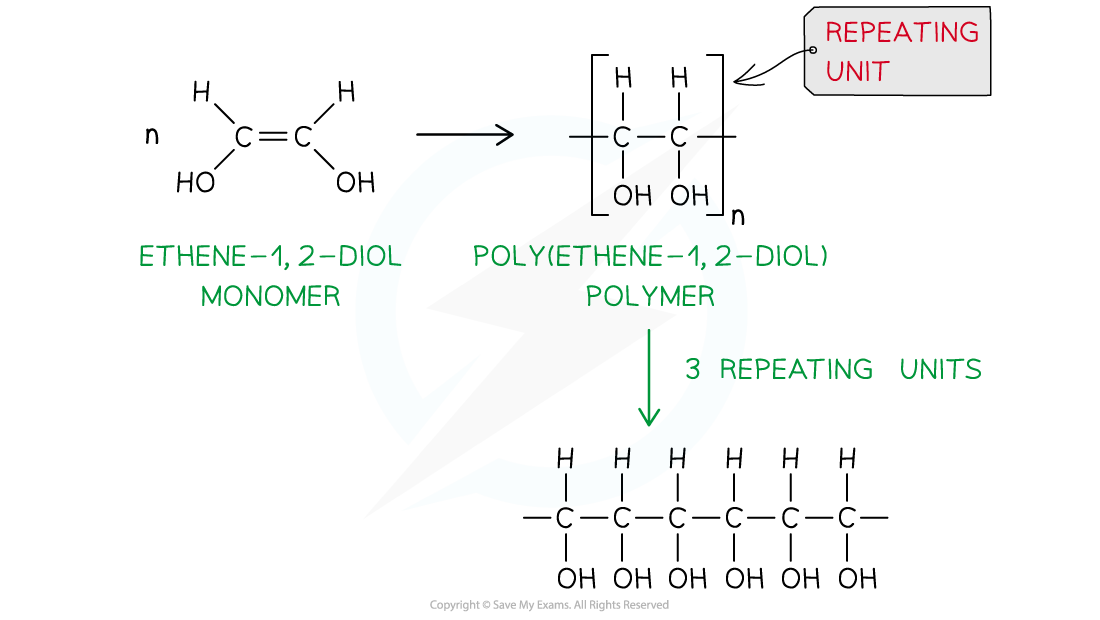
- Again, the repeating unit only has 2 carbons in the polymer chain which in this case are two carbon atoms that each contain one OH group
- Thus, when ethene-1,2-diol (CH(OH)=CH(OH)) is polymerised, the C=C double bond opens to produce a repeating unit of CH(OH)-CH(OH) which gives the polymer poly(ethene-1,2-diol)
Exam Tip
The section of the polymer chain shown inside the square brackets by the structural or displayed formula is the repeat unit and not the monomerThe monomer is the same as the repeat unit except for that it has C=C bonds instead of C-C bonds
转载自savemyexams

在线登记
最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








