- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记10.2.2 Alkanes - Halogenation
Halogenation of Alkanes
Free-radical substitution of alkanes
- Alkanes can undergo free-radical substitution in which a hydrogen atom gets substituted by a halogen (chlorine/bromine)
- Since alkanes are very unreactive, ultraviolet light (sunlight) is needed for this substitution reaction to occur
- The free-radical substitution reaction consists of three steps:
- In the initiation step, the halogen bond (Cl-Cl or Br-Br) is broken by UV energy to form two radicals
- These radicals create further radicals in a chain type reaction called the propagation step
- The reaction is terminated when two radicals collide with each other in a termination step
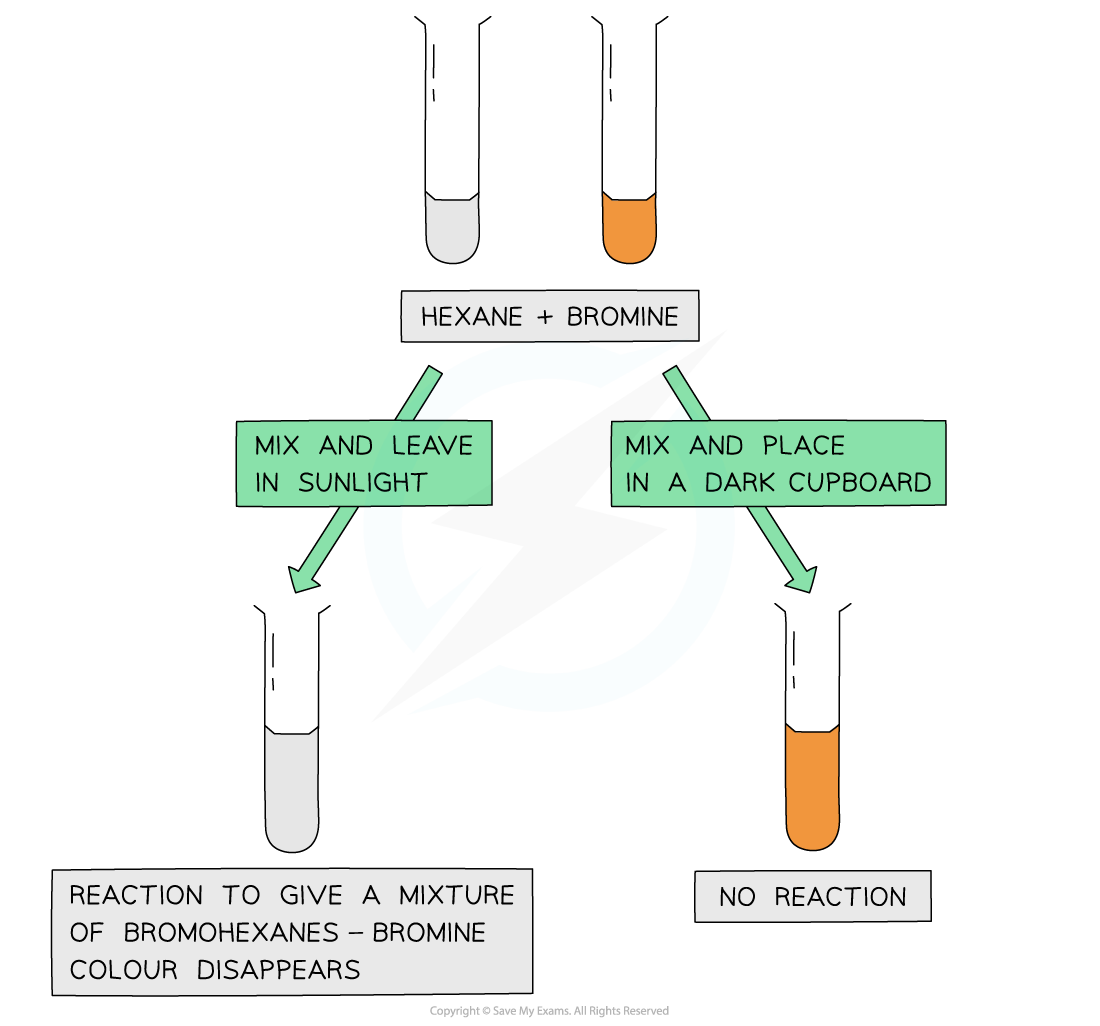
The fact that the bromine colour has disappeared only when mixed with an alkane and placed in sunlight suggests that the ultraviolet light is essential for the free radical substitution reaction to take place
Initiation step
- In the initiation step the Cl-Cl or Br-Br is broken by energy from the UV light
- This produces two radicals in a homolytic fission reaction
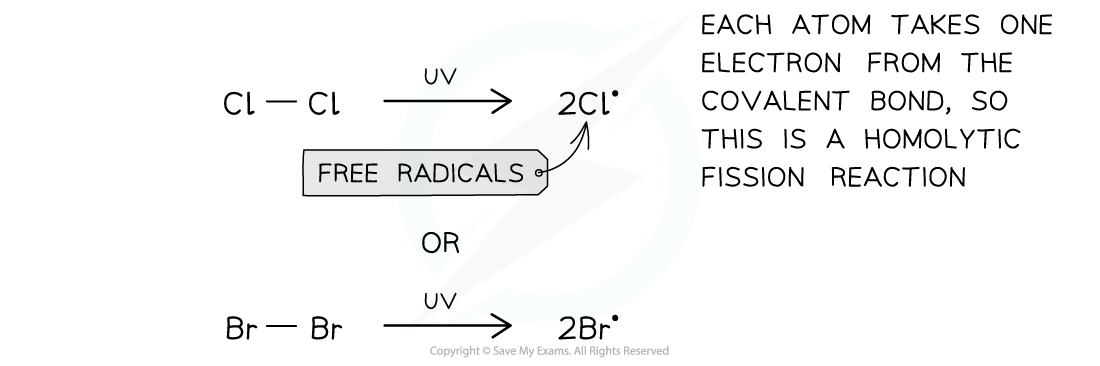
The first step of the free-radical substitution reaction is the initiation step in which two free radicals are formed by sunlight
Propagation step
- The propagation step refers to the progression (growing) of the substitution reaction in a chain type reaction
- Free radicals are very reactive and will attack the unreactive alkanes
- A C-H bond breaks homolytically (each atom gets an electron from the covalent bond)
- An alkyl free radical is produced
- This can attack another chlorine/bromine molecule to form the halogenoalkane and regenerate the chlorine/bromine free radical
- This free radical can then repeat the cycle
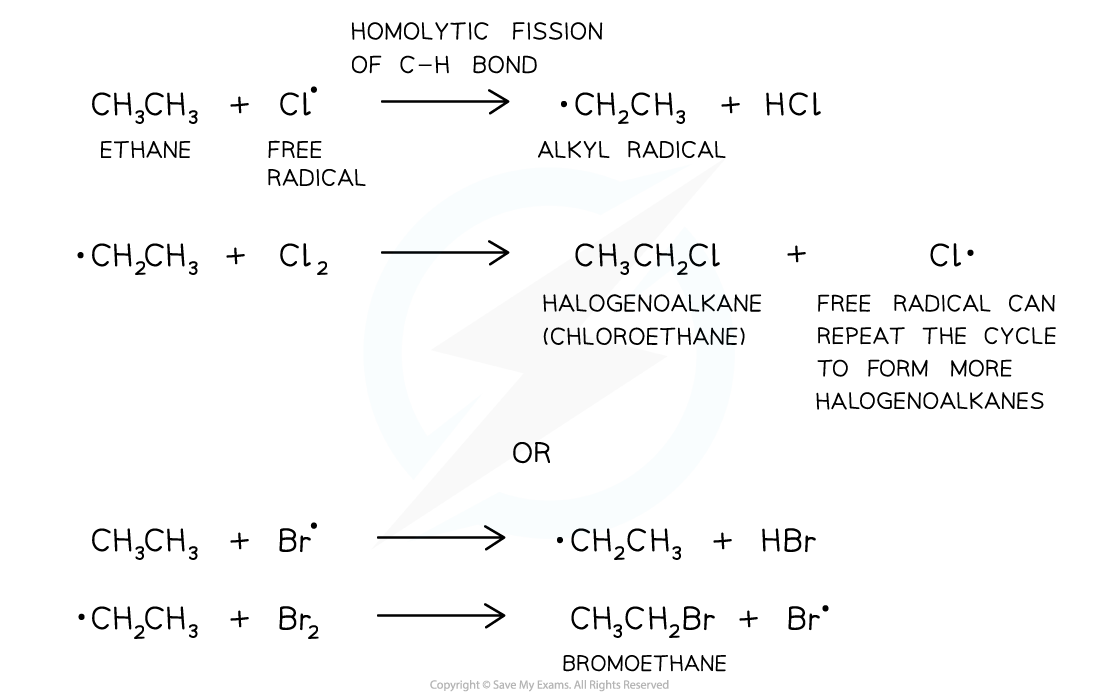
The second step of the free-radical substitution reaction is the propagation step in which the reaction grows in a chain type reaction
-
- This reaction is not very suitable for preparing specific halogenoalkanes as a mixture of substitution products are formed
- If there is enough chlorine/bromine present, all the hydrogens in the alkane will eventually get substituted (eg. ethane will become C2Cl6/C2Br6)

The free-radical substitution reaction gives a variety of products and not a pure halogenoalkane
Termination step
- The termination step is when the chain reaction terminates (stops) due to two free radicals reacting together and forming a single unreactive molecule
- Multiple products are possible
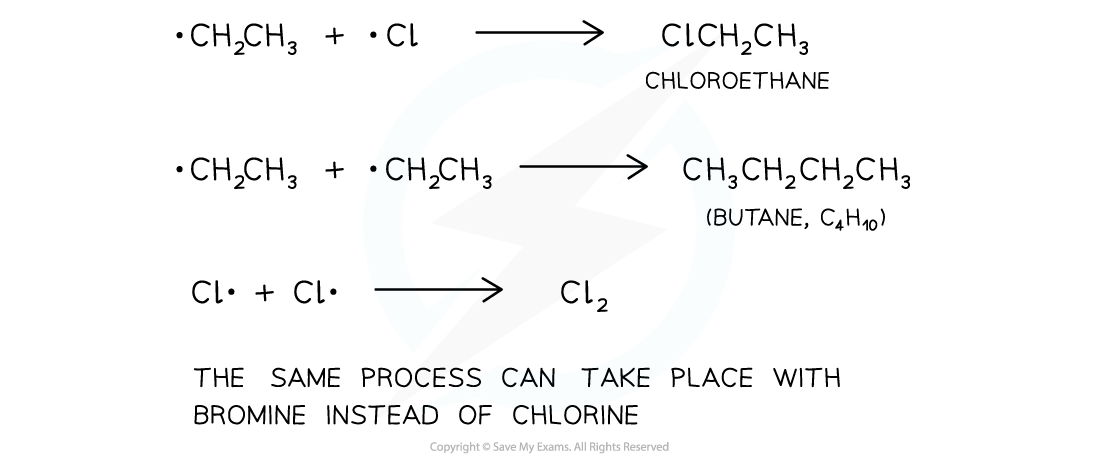
The final step in the substitution reaction to form a single unreactive molecule
Exam Tip
Make sure you practice and are able to write out these equations, especially the propagation steps which students frequently get wrong.It is quite common for students to incorrectly show a hydrogen radical produced in propagation, which does not happen:
CH3CH3+ Cl· → CH3CH2 Cl + H·
Do not fall into this trap!

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








