- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记10.1.7 Organic Families - Carbonyls
Carbonyls
- Carbonyl is the collective name for compounds containing the functional group C=O
- The general formula of a carbonyl is CnH2nO
- The two sub-families of carbonyls are aldehyde and ketone (known in some countries as alkanals and alkanones)
Aldehydes
- If the carbonyl group is on the end of a chain then it is an aldehyde and has the functional group formula, RCHO
- the H is written before the O so as not to confuse it with an alcohol
- The nomenclature of carbonyls follows the pattern alkan + al
- There is no need to use numbers in the name as aldehyde will always be on the number 1 carbon atom
Ketones
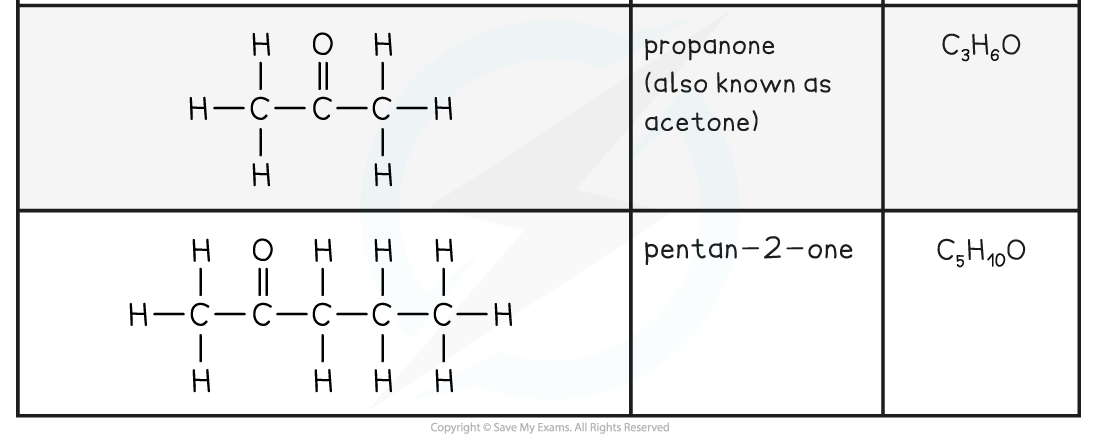
- Ketones have a minimum of three carbons and have the general functional group formula, RCOR
- The nomenclature of ketones follows the pattern alkan + one
- After butanone, the carbonyl group can have positional isomers, so numbering must be used
- For example pentan-2-one and pentan-3-one
Aldehyde and Ketone Examples Table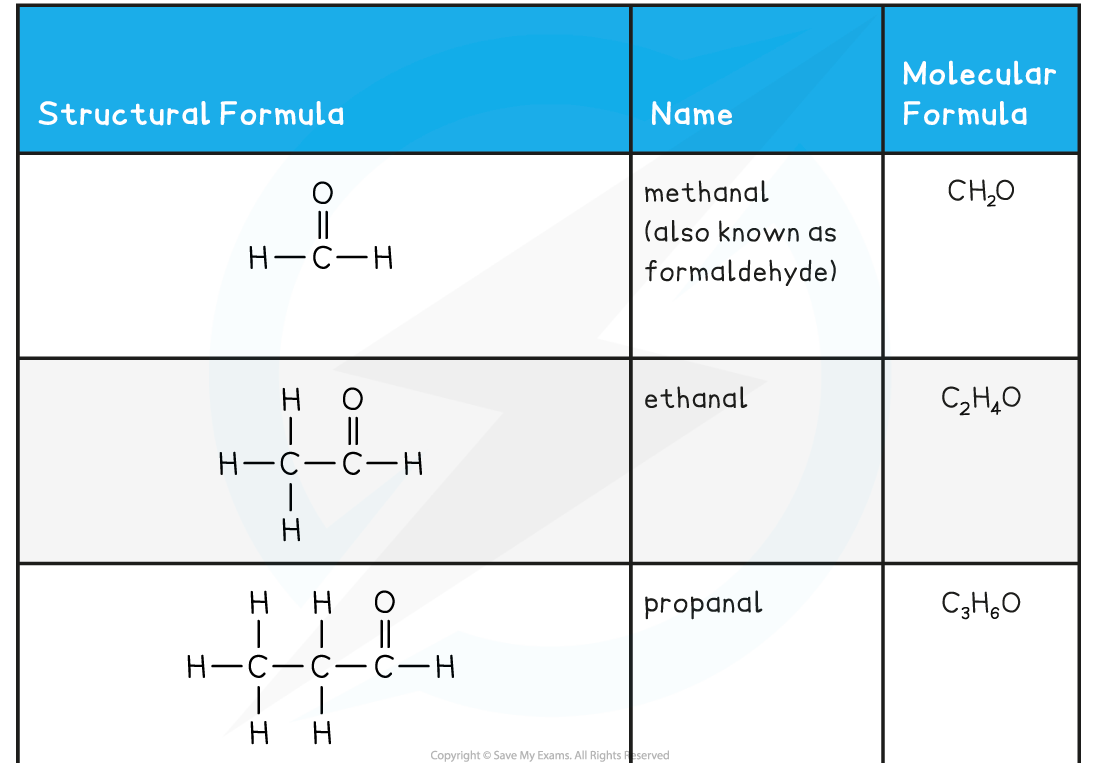
- As they have a very similar functional group arrangement, aldehydes and ketones show similar chemical reactions
- Differences in their chemistry are due to the reactions that involve the H on the aldehyde or the nature of the R group
- The difference in electronegativity between oxygen and carbon means the C=O is polar, leading to dipole-dipole attractions between the molecules which results in:
- higher than expected boiling points for small molecules
- solubility in water for the lower members of the families
- Aldehydes and ketones with the same number of carbons are functional group isomers
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









