- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记10.1.2 Understanding Organic Molecules
Representing Formulae
- Organic compounds can be represented in a number of ways:
- Empirical Formulae
- Molecular Formulae
- Structural Formulae
- Condensed Structural Formulae
- The empirical formula shows the simplest possible ratio of the atoms in a molecule
- For example:
- Hydrogen peroxide is H2O2 but the empirical formula is HO
- The molecular formula shows the actual number of atoms in a molecule
- For example:
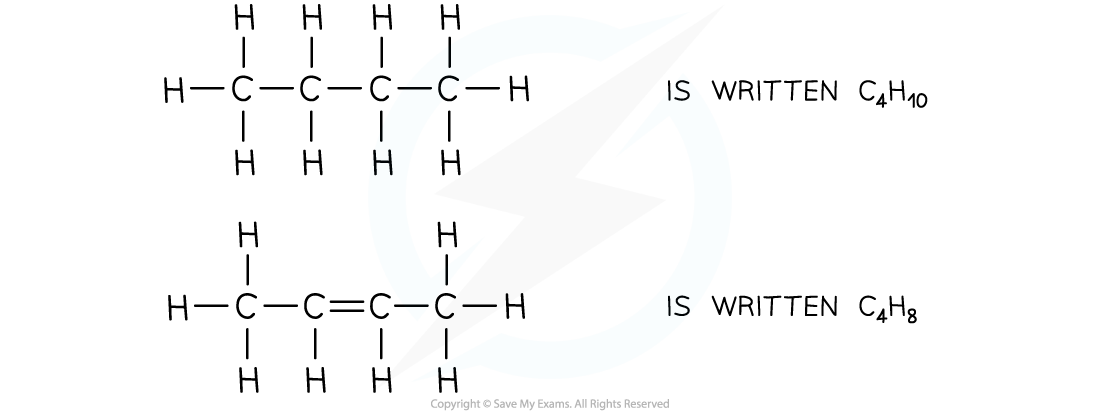
The molecular formulae of butane and butene
- The structural formula shows the spatial arrangement of all the atoms and bonds in a molecule
- This is also known as the displayed formula or graphical formula.
- For example:
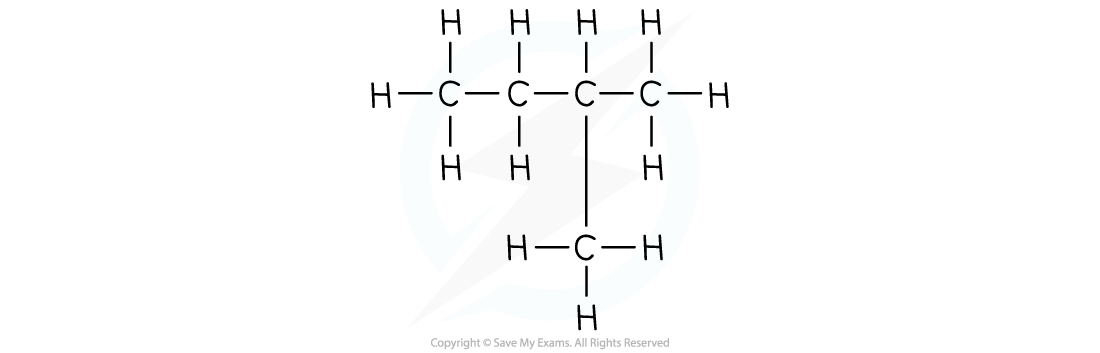
The structural formula of 2-methylbutane
- In a condensed structural formulae enough information is shown to make the structure clear, but most of the actual covalent bonds are omitted
- Only important bonds are always shown, such as double and triple bonds
- Identical groups can be bracketed together
- Side groups are also shown using brackets
- Straight chain alkanes are shown as follows:

Representing condensed structural formulae of straight chains
- Branched alkanes are shown as follows:

Representing condensed structural formulae of branched alkanes
- Alkenes are shown as follows:

Representing condensed structural formulae of alkenes
Isomers
- Structural isomers are compounds that have the same molecular formula but different structural formulae
- Eg. propene and cyclopropane
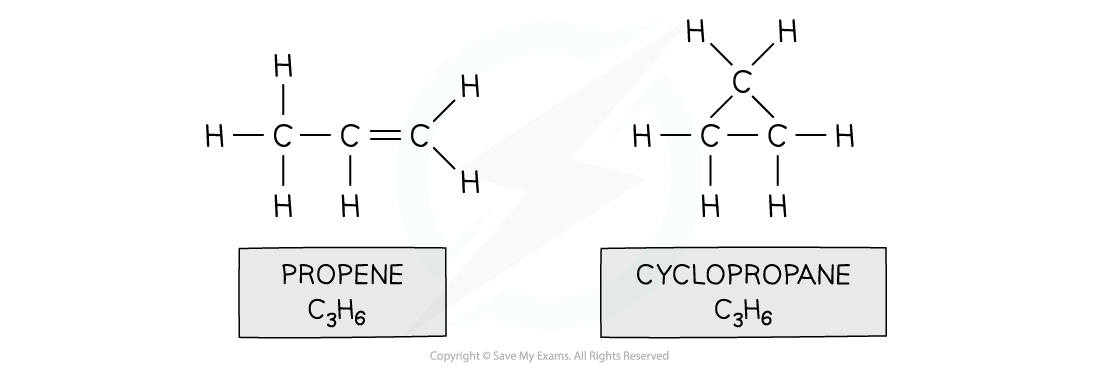
Both propene and cyclopropane are made up of 3 carbon and 6 hydrogen atoms but the structure of the two molecules differs
- There are three different types of structural isomerism:
- Branch-Chain isomerism
- Positional isomerism
- Functional group isomerism
Branch-Chain isomerism
- Branch-Chain isomerism is when compounds have the same molecular formula, but their longest hydrocarbon chain is not the same
- This is caused by branching
- Eg. pentane and 2,2-dimethylpropane
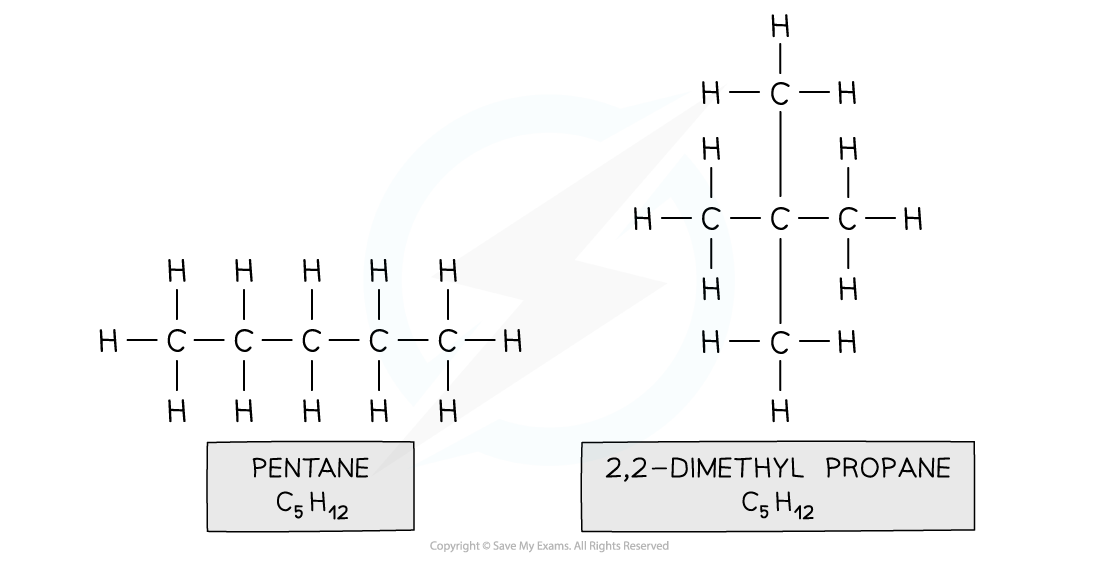
Both compounds are made up of the same atoms however the longest carbon chain in pentane is 5 and in 2,2-dimethylpropane it is 3 (with two methyl branches)
Positional isomerism
- Positional isomers arise from differences in the position of a functional group in each isomer
- The functional group can be located on different carbons
- For example, butan-1-ol and butan-2-ol
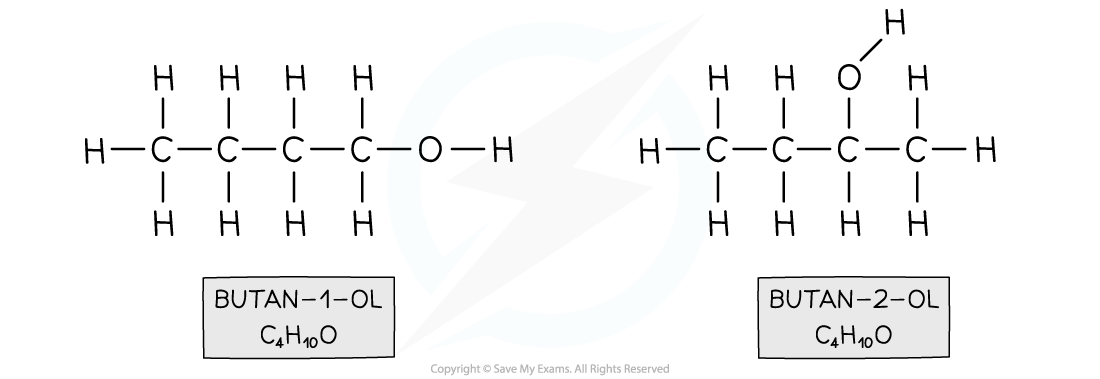
Both compounds have an alcohol group and are made up of 4 carbon, 10 hydrogen and one oxygen atom however in butan-1-ol the functional group is located on the first carbon and in butan-2-ol on the second carbon
Functional group isomeris
- When different functional groups result in the same molecular formula, functional group isomers arise
- The isomers have very different chemical properties as they have different functional groups
- For example, butanol and ethoxyethane
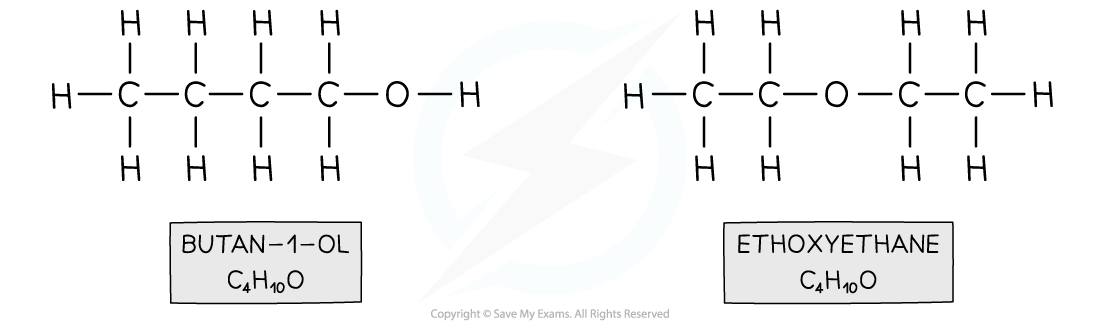
Both compounds have the same molecular formula however butan-1-ol contains an alcohol functional group and ethoxyethane an ether functional group
- You should be able to deduce all possible isomers for organic compounds knowing their molecular formula
Worked Example
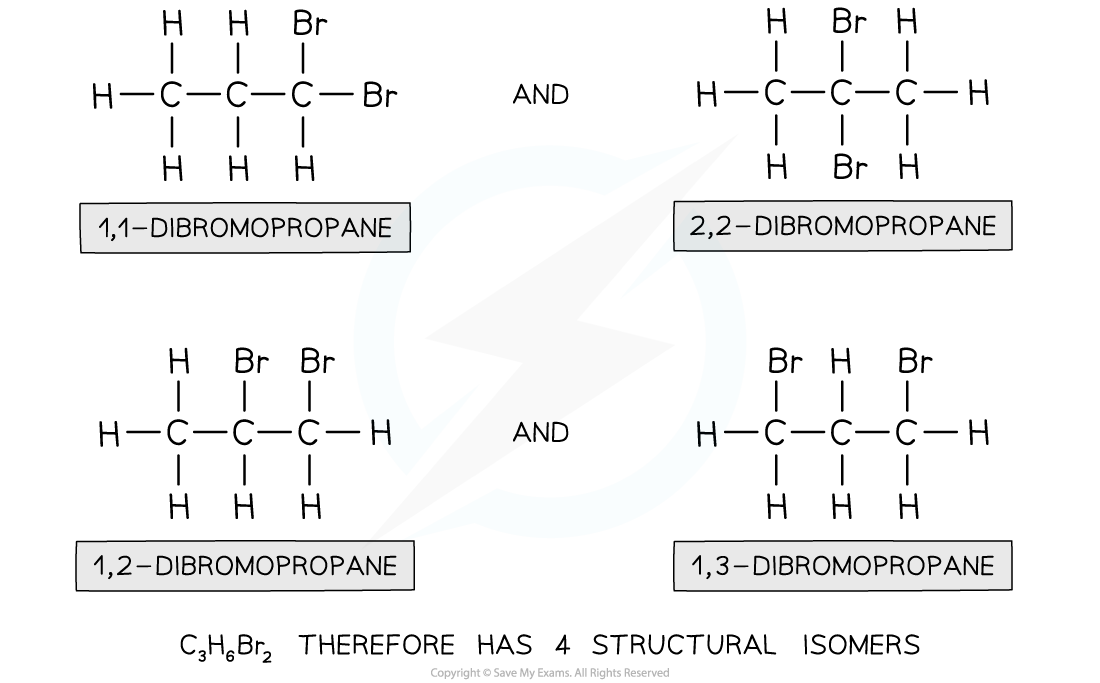
How many isomers are there of, C3H6Br2 ?
Answer:
Step 1: Draw the structural formula of the compound
 Step 2: Determine whether there is functional group, branch-chain or positional isomerism
Step 2: Determine whether there is functional group, branch-chain or positional isomerism
-
- Functional group? No, as Br is the only functional group possible
- Branch-chain? No, as the longest chain can only be 3
- Positional? Yes, as the two bromine atoms can be bonded to different carbon atoms

Worked Example
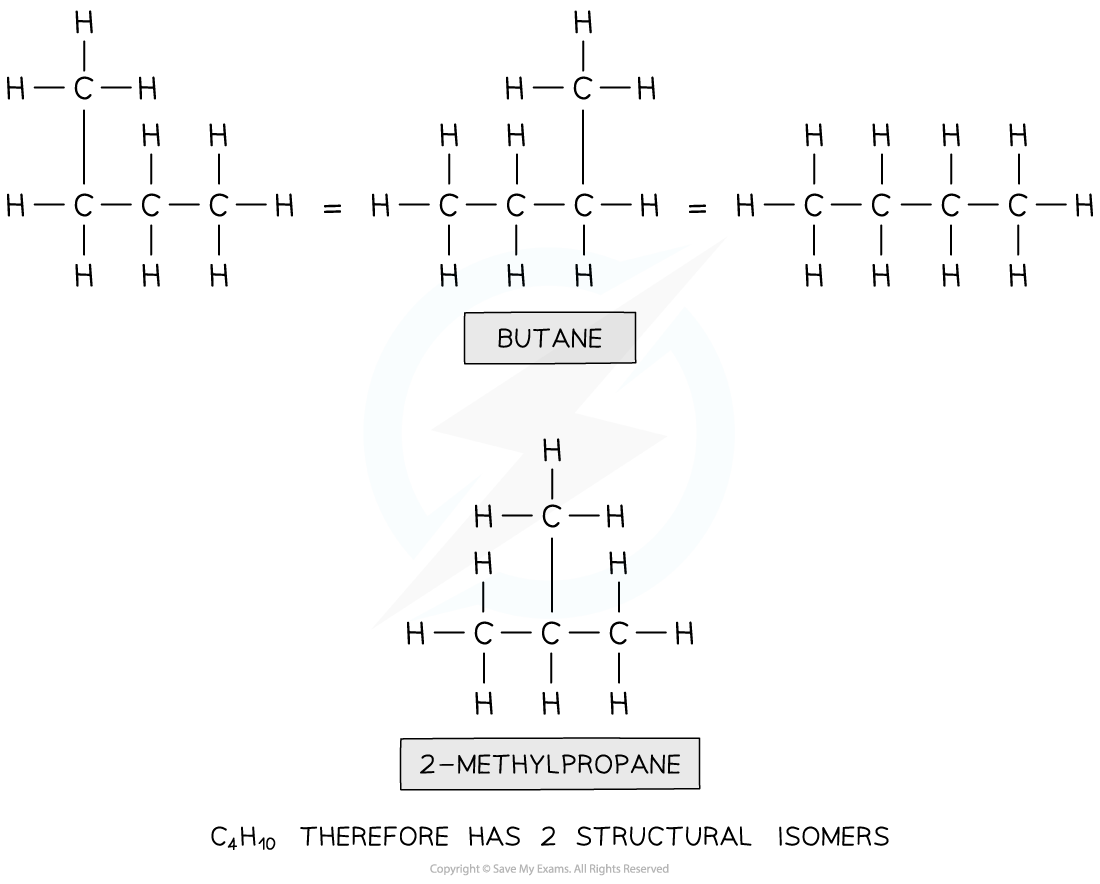
How many isomers are there of the compound with molecular formula C4H10 ?
Answer:
Step 1: Draw the structural formula of the compound

Step 2: Determine whether it is a functional group, chain or positional isomerism
-
- Functional group? No, as there are no functional groups
- Positional? No, as there are no functional groups which can be positioned on different carbon atoms
- Chain? yes!
Exam Tip
Don't be fooled by molecules by bending and turning through 90 degrees - that does not make them isomers. The best test is to try and name them - isomers will have a different name.
Saturated & Unsaturated
Saturated & unsaturated hydrocarbons
- Saturated hydrocarbons are hydrocarbons which contain single bonds only resulting in the maximum number of hydrogen atoms in the molecule
- Unsaturated hydrocarbons are hydrocarbons which contain carbon-carbon double or triple bonds
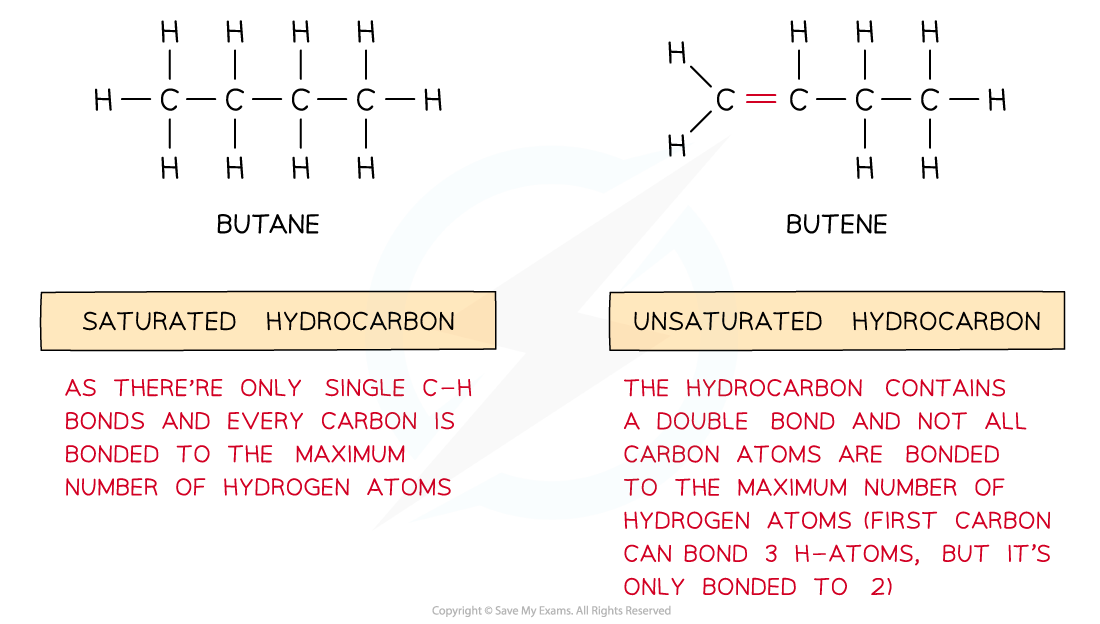
The diagram shows saturated hydrocarbons which contain single bonds only and unsaturated hydrocarbons which contain double/triple bonds as well
转载自savemyexams

在线登记
最新发布
翰林课程体验,退费流程快速投诉邮箱: yuxi@linstitute.net 沪ICP备2023009024号-1








