- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记9.1.10 Electrolytic Cells
Electrolytic Cells
- An ionic compound conducts electricity when it is molten or in solution
- The current causes the ionic compound to split up and form new substances.
- This process is called electrolysis, a word which comes from Greek and means “splitting by electricity”
- Electrolysis has many uses, including:
- purifying copper
- plating metals with silver and gold
- extracting reactive metals, such as aluminium
- making chlorine, hydrogen and sodium hydroxide
Electrolysis cells
- Electrolysis cells can be constructed using a beaker or crucible as the cell depending whether the ionic compound is in solution or molten
- For Standard Level Chemistry we only need to look at the electrolysis of molten ionic compounds
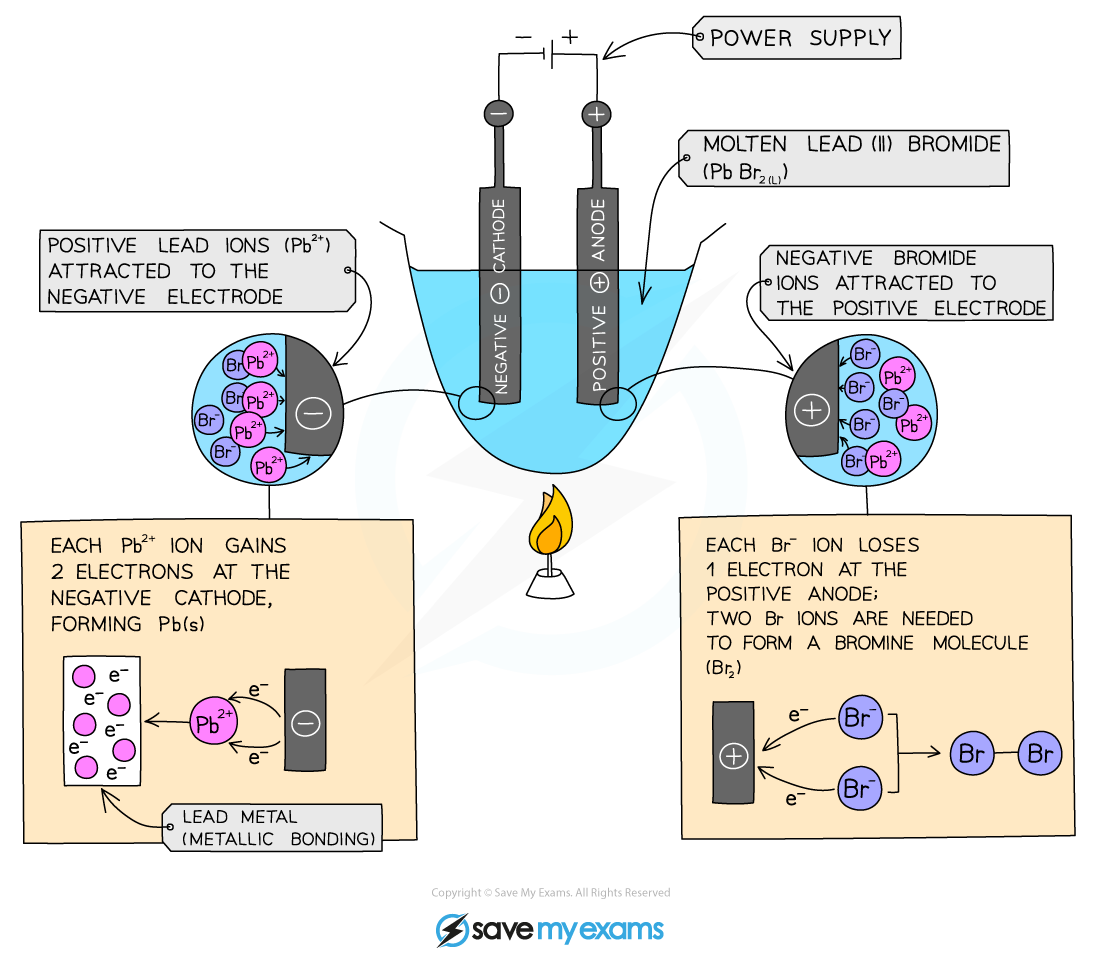
Electrolysis of a molten ionic compound
- In electrolysis, the substance that the current passes through and splits up is called the electrolyte
- The electrolyte contains positive and negative ions
- What happens to these ions during electrolysis?
- Negative ions move to the anode and lose electrons - this is oxidation
- Positive ions move to the cathode and gain electrons - this is reduction
- Electrically neutral atoms or molecules are released
Electrolysis of molten lead bromide
- The reactions which take place at the electrodes can be shown by half equations
- When the positive lead ions move to the cathode, they gain electrons in a reduction reaction:
Pb2+(aq) + 2e- ⇌ Pb(s)
- Similarly when the negative bromide ions move to the anode they lose electrons in an oxidation reaction:
2Br-(l) - 2e- ⇌ Br2 (l)
- Sometimes oxidation reactions are written with '+2e-' on the right of the arrow instead of'-2e' on the left
- In this case the alternative half equation is:
2Br-(l) ⇌ Br2 (l) + 2e-
- Since metals are always cations and non-metal anions, it is easy to predict the products of electrolysis of molten salts:
- Metals will always be formed at the cathode and non-metals at the anode
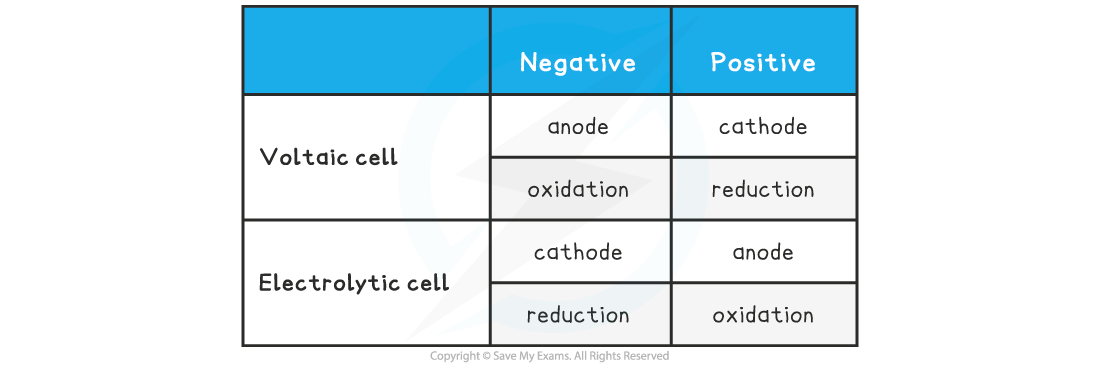
Comparing Voltaic & Electrolytic Cells Summary Table
Exam Tip
Rather confusingly........in electrolytic cells the negative electrode is called the cathode and the positive electrode is called the anode, which is the opposite to voltaic cellsThis naming confusion arises because in both cases the cathode is where reduction occurs and the anode is where oxidation occursTo avoid this confusion many people only use the words cathode and anode when talking about electrolysis and use negative and positive electrodes when talking about voltaic cells
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








