- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记9.1.3 Oxidizing & Reducing agents
Oxidizing & Reducing Agents
Oxidising agent
- An oxidising agent is a substance that oxidises another atom or ion by causing it to lose electrons
- An oxidising agent itself gets reduced – gains electrons
- Therefore, the ox. no. of the oxidising agent decreases

Example of an oxidising agent in a chemical reaction
Reducing agent
- A reducing agent is a substance that reduces another atom or ion by causing it to gain electrons
- A reducing agent itself gets oxidised – loses/donates electrons
- Therefore, the ox. no. of the reducing agent increases

Example of a reducing agent in a chemical reaction
- For a reaction to be recognised as a redox reaction, there must be both an oxidising and reducing agent
- Some substances can act both as oxidising and reducing agents
- Their nature is dependent upon what they are reacting with and the reaction conditions
Oxidising & Reducing Agents Table

Identifying Oxidizing & Reducing Agents
- Applying the definitions of oxidising and reducing agents allows you to identify them in chemical equations
- By deducing the oxidation numbers of the species you can determine whether it has been oxidised or reduced
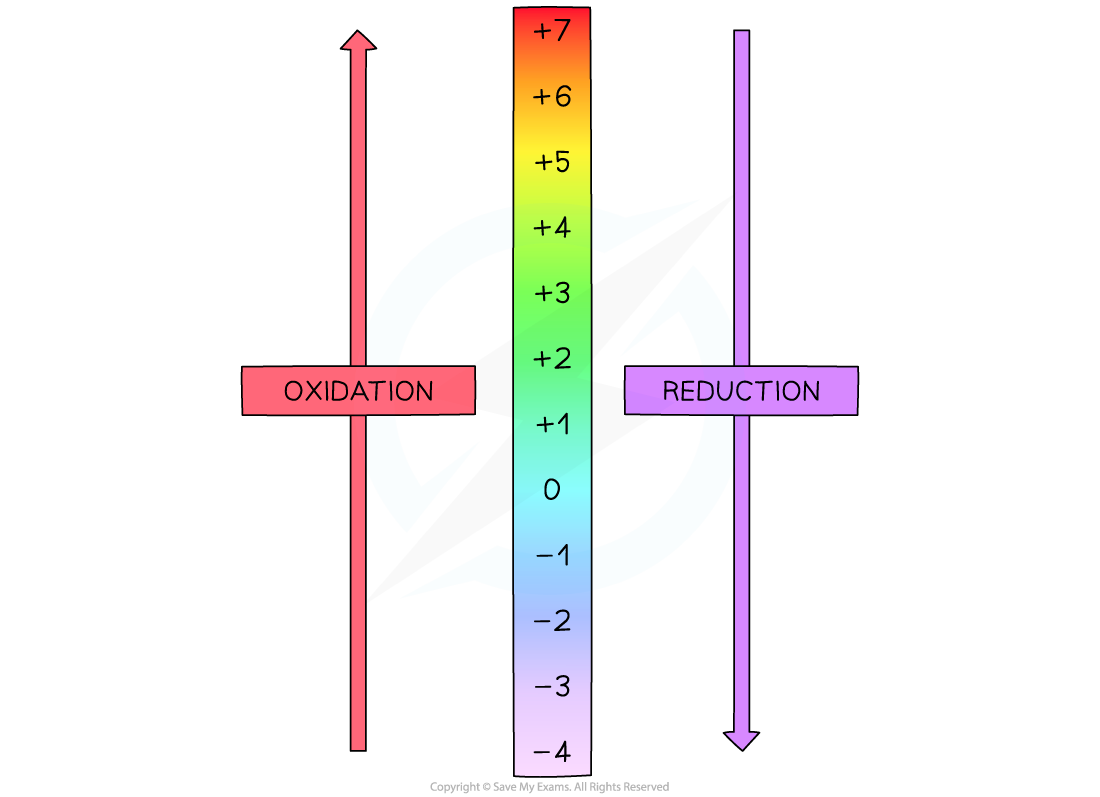
A visual reminder of oxidation numbers and redox. This is like an elevator in a building going up to higher floors is oxidation and going down to the basement is reduction
Worked Example
Four reactions are shown. In which reaction is the species in blue acting as an oxidising agent?
A. Cr2O72- + 8H+ + 3SO32- → 2Cr3+ + 4H2O+ 3SO42-
B. Mg + Fe2+ → Mg2+ + Fe
C. Cl2 + 2Br- → 2Cl- + Br2
D. Fe2O3 + 3CO → 2Fe + 3CO2
Answer:
The correct option is B.
-
- Oxidising agents are substances that oxidise other species, gain electrons and are themselves reduced.
- Write down the oxidation numbers of each species in the reaction
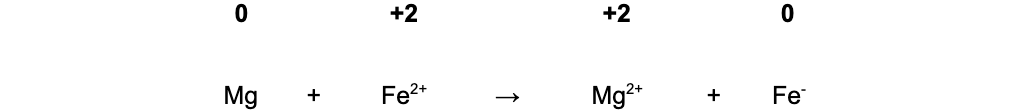
-
- In equation B, Fe2+ oxidises Mg(0) to Mg2+(+2) and is itself reduced from Fe2+(+2) to Fe(0)
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









