- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记8.2.1 Acid-base Titrations
Acid-Base Titrations
- The steps involved in performing a titration and titration calculation are outlined in Topic 1.2.9 Titrations
- Acid-base titrations follow the same steps and are used to find the unknown concentrations of solutions of acids and bases
- Acid-base indicators give information about the change in chemical environment
- They change colour reversibly depending on the concentration of H+ ions in the solution
- Indicators are weak acids and bases where the conjugate bases and acids have a different colour
- Many acid-base indicators are derived from plants, such as litmus
Common Indicators Table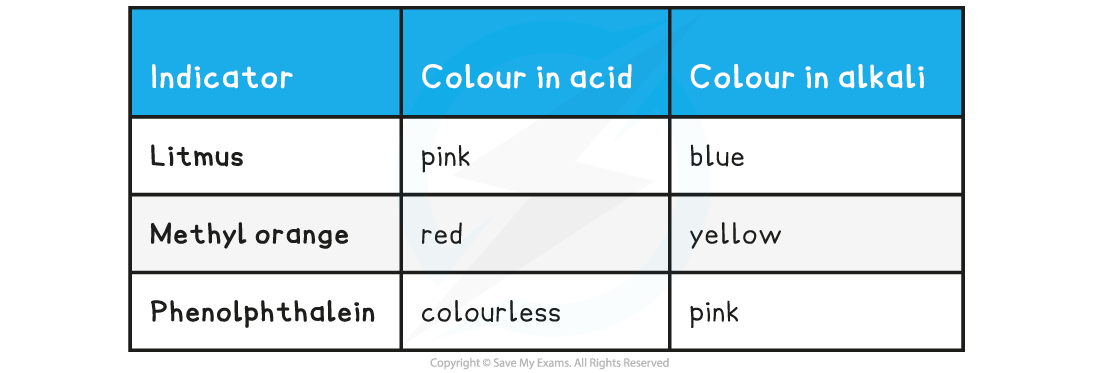
- A good indicator gives a very sharp colour change at the equivalence point
- In titrations is it not always possible to use two colour indicators because of this limitation, so for example litmus cannot be used successfully in a titration
- When phenolphthalein is used, it is usually better to have the base in the burette because it is easier to see the sudden and permanent appearance of a colour (pink in this case) than the change from a coloured solution to a colourless one
Exam Tip
Make sure you learn the colours of the common acid-base indicato
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1