- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记8.1.3 Characteristic Reactions of Acids
Characteristic Reactions of Acids
Metals and acids
- The typical reaction of a metal and an acid can be summarized as
acid + metal → salt + hydrogen
- For example:
2HCl (aq) + Zn (s) → ZnCl2 (aq) + H2 (g)
hydrochloric acid + zinc → zinc chloride + hydrogen
- Clearly, the extent of reaction depends on the reactivity of the metal and the strength of the acid
- Very reactive metals would react dangerously with acids and these reactions are not usually carried out
- Metals low in reactivity do not react at all, for instance copper does not react with dilute acids
- Stronger acids will react more vigorously with metals than weak acids. What signs of reaction would be expected to be different between the two?
- Faster reaction, seen as
- more effervescence
- the metal dissolves faster
- More exothermic
- Faster reaction, seen as
Metals and oxides
- The reaction of an acid with a metal oxide forms two products:
acid + metal oxide → salt + water
- For example:
2HCl (aq) + CaO (s) → CaCl2 (aq) + H2O (l)
hydrochloric acid + calcium oxide → calcium chloride + water
Metals and hydroxides
- The reaction with a metal hydroxide and an acid follows the same pattern as an oxide:
acid + metal hydroxide → salt + water
- A suitable example might be:
H2SO4 (aq) + Mg(OH)2 (s) → MgSO4 (aq) + 2H2O (l)
sulfuric acid + magnesium hydroxide → magnesium sulfate + water
Metals and carbonates
- The reaction between a metal carbonate and an acid produces three products:
acid + metal carbonate → salt + water + carbon dioxide
- For example:
2HNO3 (aq) + CuCO3 (s) → Cu(NO3)2 (aq) + H2O (l) + CO2 (g)
nitric acid + copper carbonate → copper nitrate + water + carbon dioxide
Metals and hydrogencarbonates
- The reaction between a metal hydrogencarbonate and an acid is the same as the carbonate reaction with a slight difference in stoichiometry:
acid + metal hydrogencarbonate → salt + water + carbon dioxide
- An example of this would be:
HCl (aq) + NaHCO3 (s) → NaCl (aq) + H2O (l) + CO2 (g)
hydrochloric acid + sodium hydrogencarbonate → sodium chloride + water + carbon dioxide
Exam Tip
Make sure you learn the formulae of the common acids and bases and that you can write examples of balanced equations of their characteristic reactions
Making Salts
- The acids and bases needed to make different salts can be deduced using the principles covered in the previous section
- The table below summarises these reactions
Making Salts Table
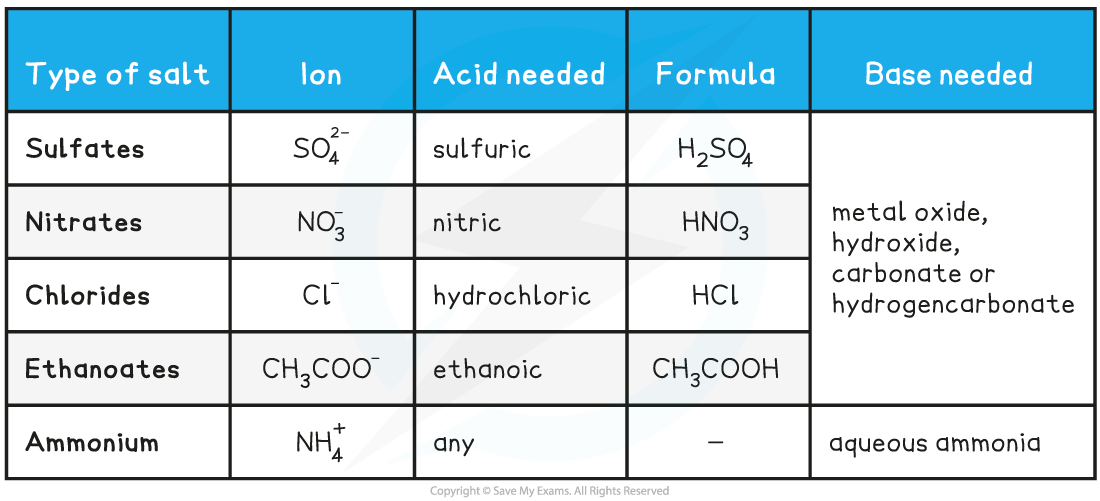
- Note that although some metals can be used to make salts, they are not classified as bases as water is not a product of the reaction
Worked Example
Which are the products of the reaction between zinc oxide and hydrochloric acid?
A. zinc chloride and carbon dioxide
B. zinc chloride, hydrogen gas and water
C. zinc, hydrogen gas and water
D. zinc chloride and water
Answer:
The correct option is D.
-
- Metal oxides when reacting with acids produce a salt and water as the only products
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








