- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记8.1.1 Brønsted–Lowry Acids & Bases
Brønsted–Lowry Acids & Bases
- The Brønsted-Lowry Theory defines acids and bases in terms of proton transfer between chemical compounds
- A Brønsted-Lowry acid is a species that gives away a proton (H+)
- A Brønsted-Lowry base is a species that accepts a proton (H+) using its lone pair of electrons
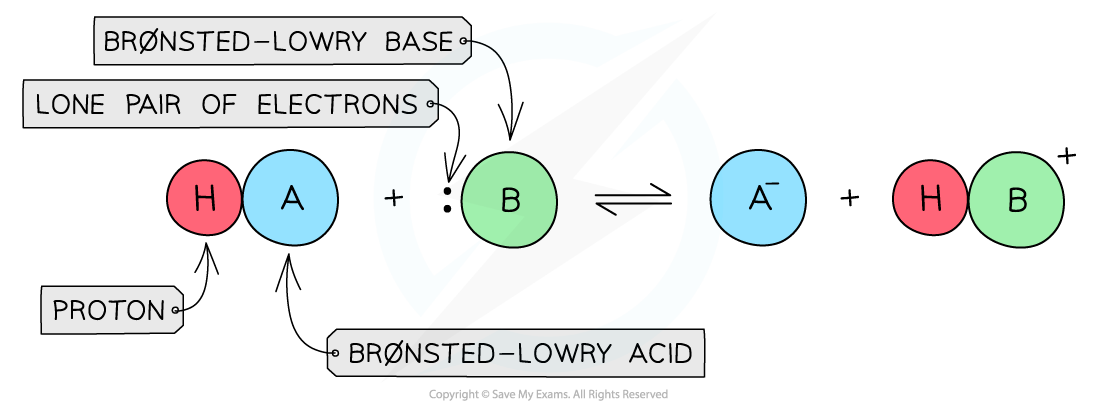
The diagram shows a Brønsted-Lowry acid which donates the proton to the Brønsted-Lowry base that accepts the proton using its lone pair of electrons
- The Brønsted-Lowry Theory is not limited to aqueous solutions only and can also be applied to reactions that occur in the gas phase
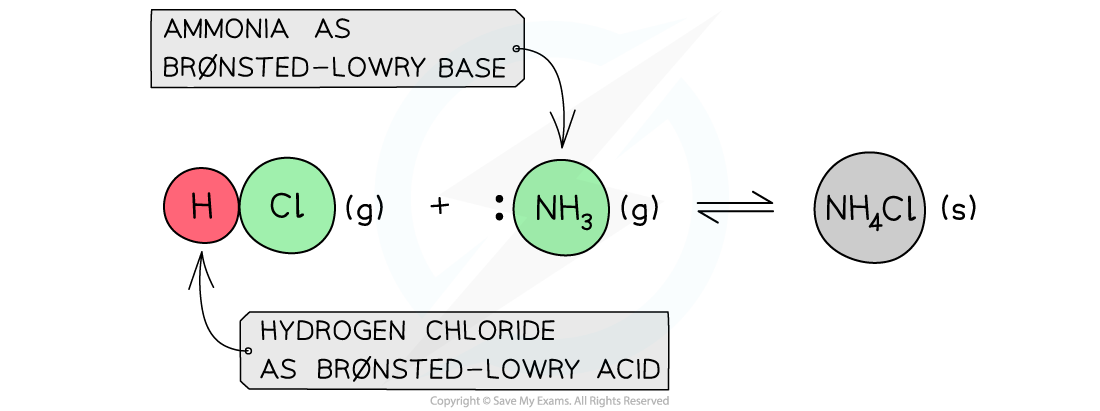
Example of a Brønsted-Lowry acid and base reaction in the gas state
Worked Example
Identify the correct role of the species in the following reaction:
H2PO4−(aq) + H2O(l) → HPO42−(aq) + H3O+(aq)
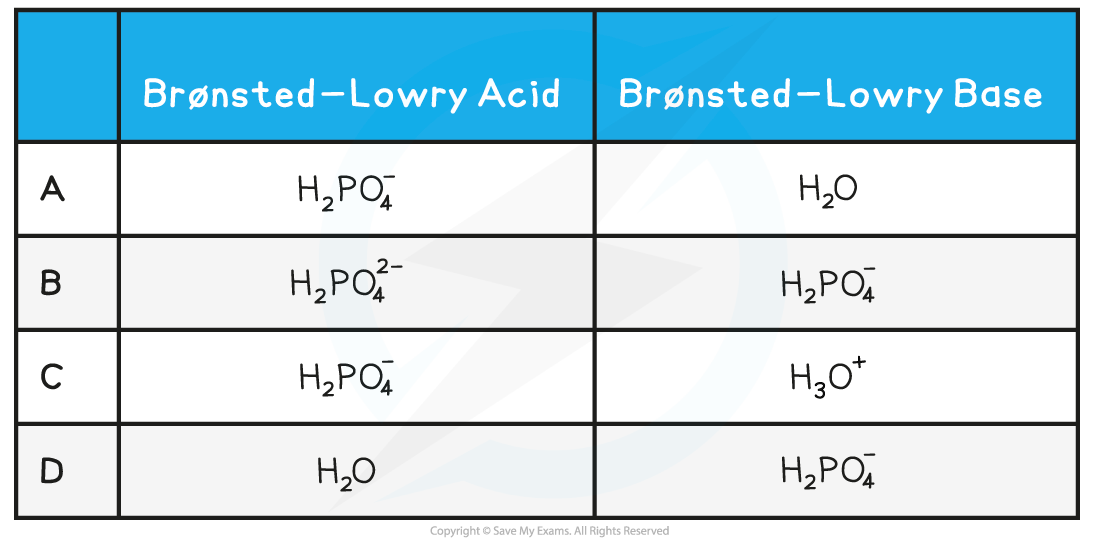
Answer:
The correct option is A.
-
- H2PO4− is donating a proton to H2O, so H2PO4− must be an acid and H2O must be a base
Exam Tip
An atom of hydrogen contains 1 proton, 1 electron and 0 neutrons. When hydrogen loses an electron to become H+ only a proton remains, which is why a H+ ion is also called a proton.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









