- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记7.1.1 The State of Equilibrium
The State of Equilibrium
Reversible reaction
- Some reactions go to completion where the reactants are used up to form the products and the reaction stops when all of the reactants are used up
- In reversible reactions the products can react to reform the original reactants
- To show a reversible reaction, two half arrows are used: ⇌
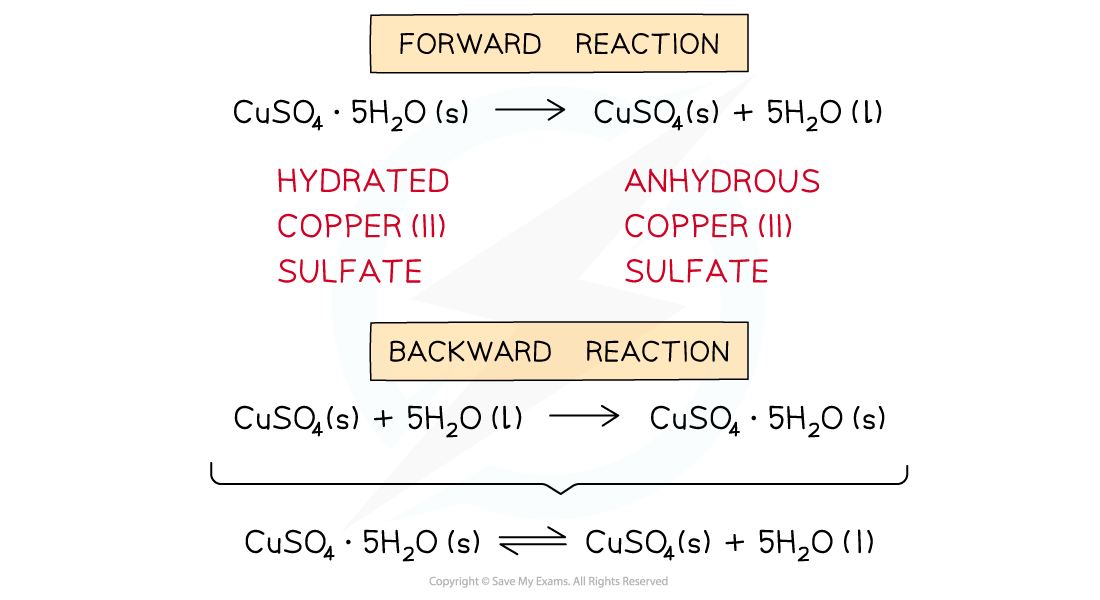
The diagram shows an example of a forward and backward reaction that can be written as one equation using two half arrows
Dynamic equilibrium
- In a dynamic equilibrium the reactants and products are dynamic (they are constantly moving)
- In a dynamic equilibrium the rate of the forward reaction is the same as the rate of the backward reaction in a closed system and the concentrations of the reactants and products is constant
- There is no change in macroscopic properties such as colour and density as they depend on concentration
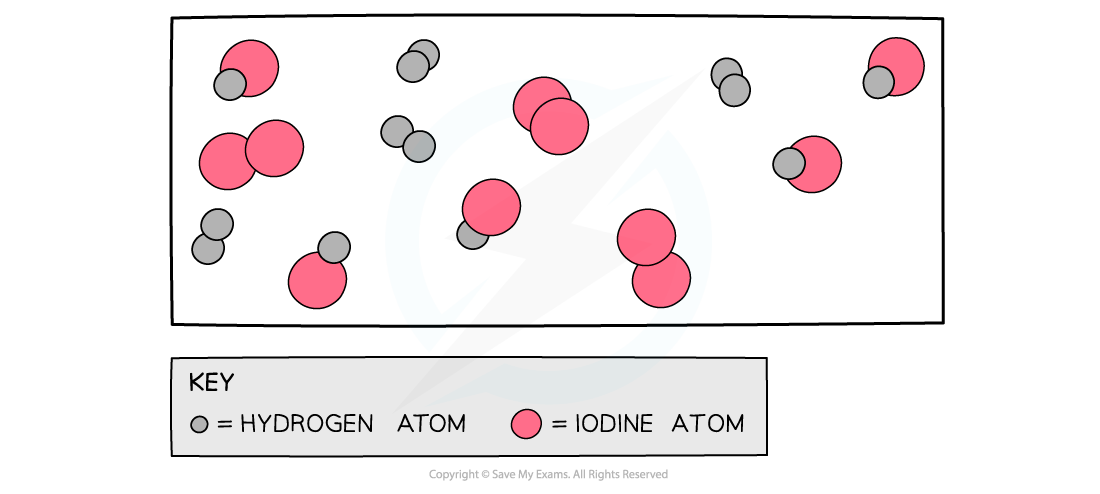
The diagram shows a snapshot of a dynamic equilibrium in which molecules of hydrogen iodide are breaking down to hydrogen and iodine at the same rate as hydrogen and iodine molecules are reacting together to form hydrogen iodide
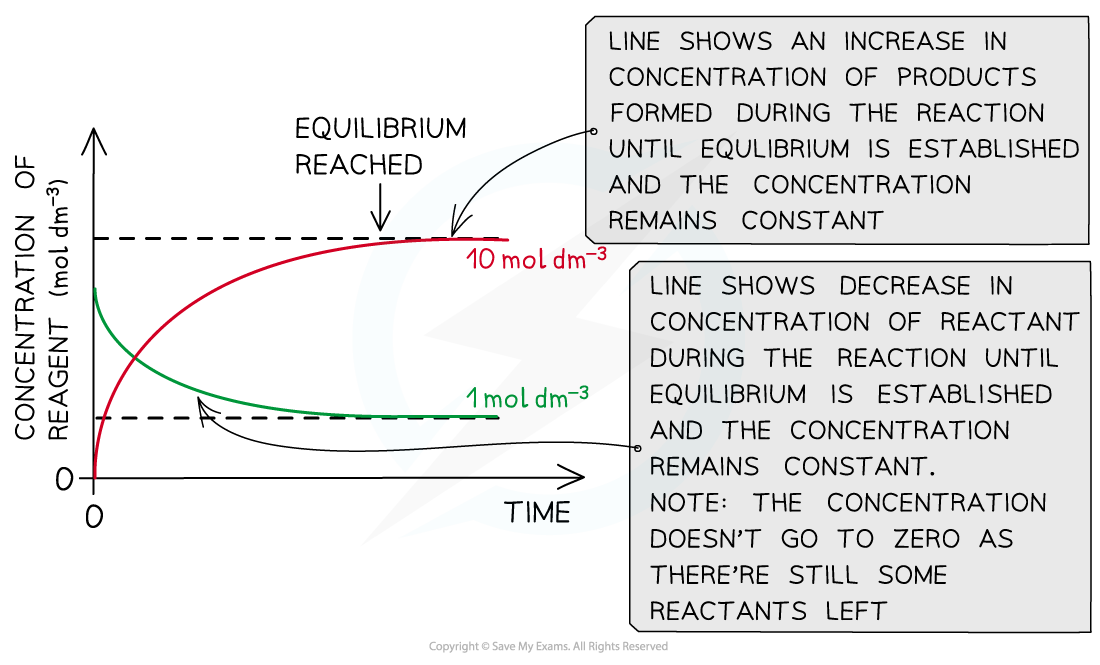
The diagram shows that the concentration of the reactants and products does not change anymore once equilibrium has been reached (equilibrium was approached using reactants)
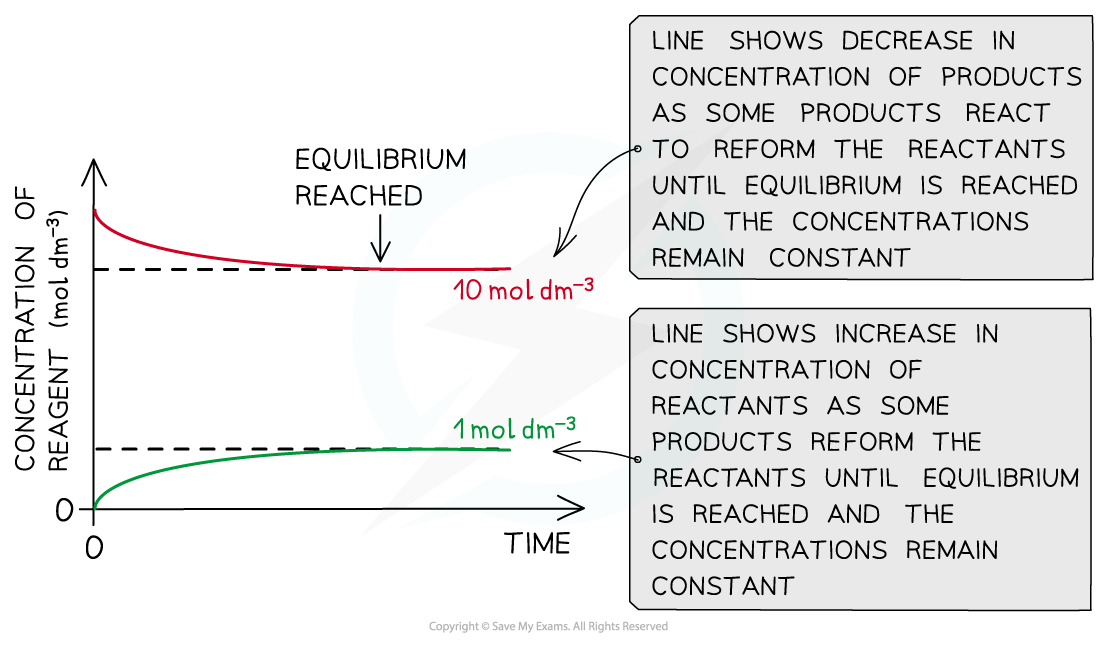
The same equilibrium can be approached starting with the products
- A closed system is one in which none of the reactants or products escape from the reaction mixture
- In an open system some matter is lost to the surroundings
- When a reaction takes place entirely in solution, equilibrium can be reached in open flasks
- If the reaction involves gas, equilibrium can only be reached in a closed system
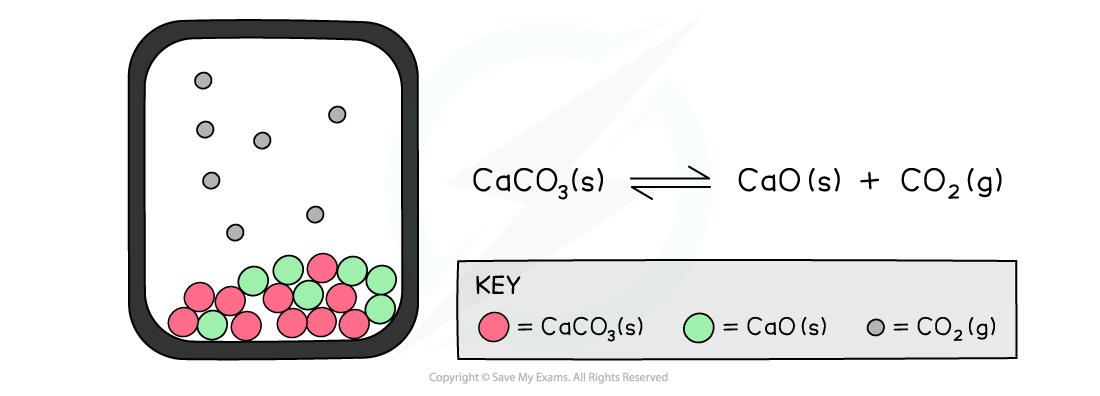
The diagram shows a closed system in which no carbon dioxide gas can escape and the calcium carbonate is in equilibrium with the calcium oxide and carbon dioxide
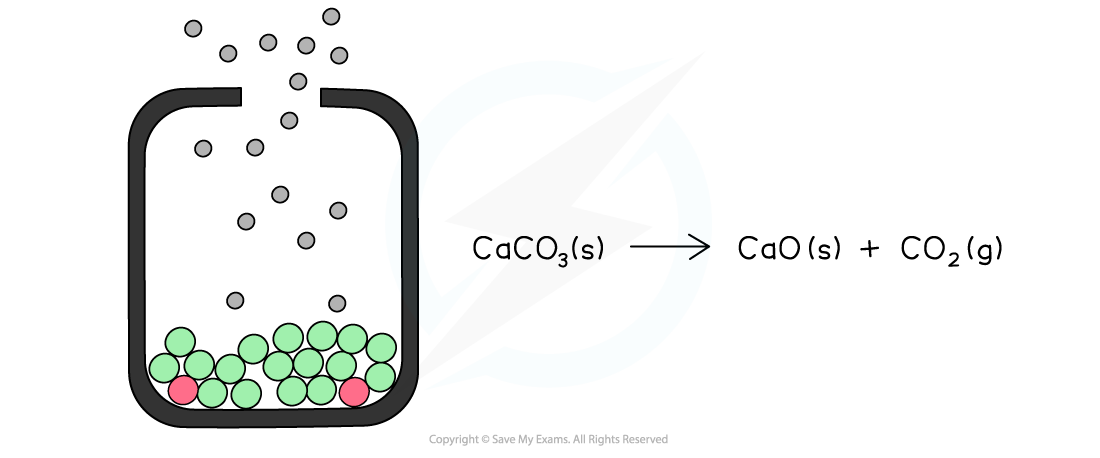
The diagram shows an open system in which the calcium carbonate is continually decomposing as the carbon dioxide is lost causing the reaction to eventually go to completion
Exam Tip
A common misconception is to think that the concentrations of the reactants and products is equal, however, they are not equal but constant (the concentrations are not changing).The dynamic equilibrium can be reached by starting either with the reactants or products.In both cases, the concentrations of the reactants and products remain constant once dynamic equilibrium has been reached.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









