- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记6.1.8 Energy Profiles & Catalysis
Energy Profiles & Catalysis
- Catalysis is the process in which the rate of a chemical reaction is increased, by adding a substance called a catalyst
- A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation energy than the uncatalysed reaction
- Catalysts can be divided into two types:
- Homogeneous catalysts
- Heterogeneous catalysts
- Homogeneous means that the catalyst is in the same phase as the reactants
- For example, the reactants and the catalysts are all liquids
- Heterogeneous means that the catalyst is in a different phase to the reactants
- For example, the reactants are gases but the catalyst used is a solid
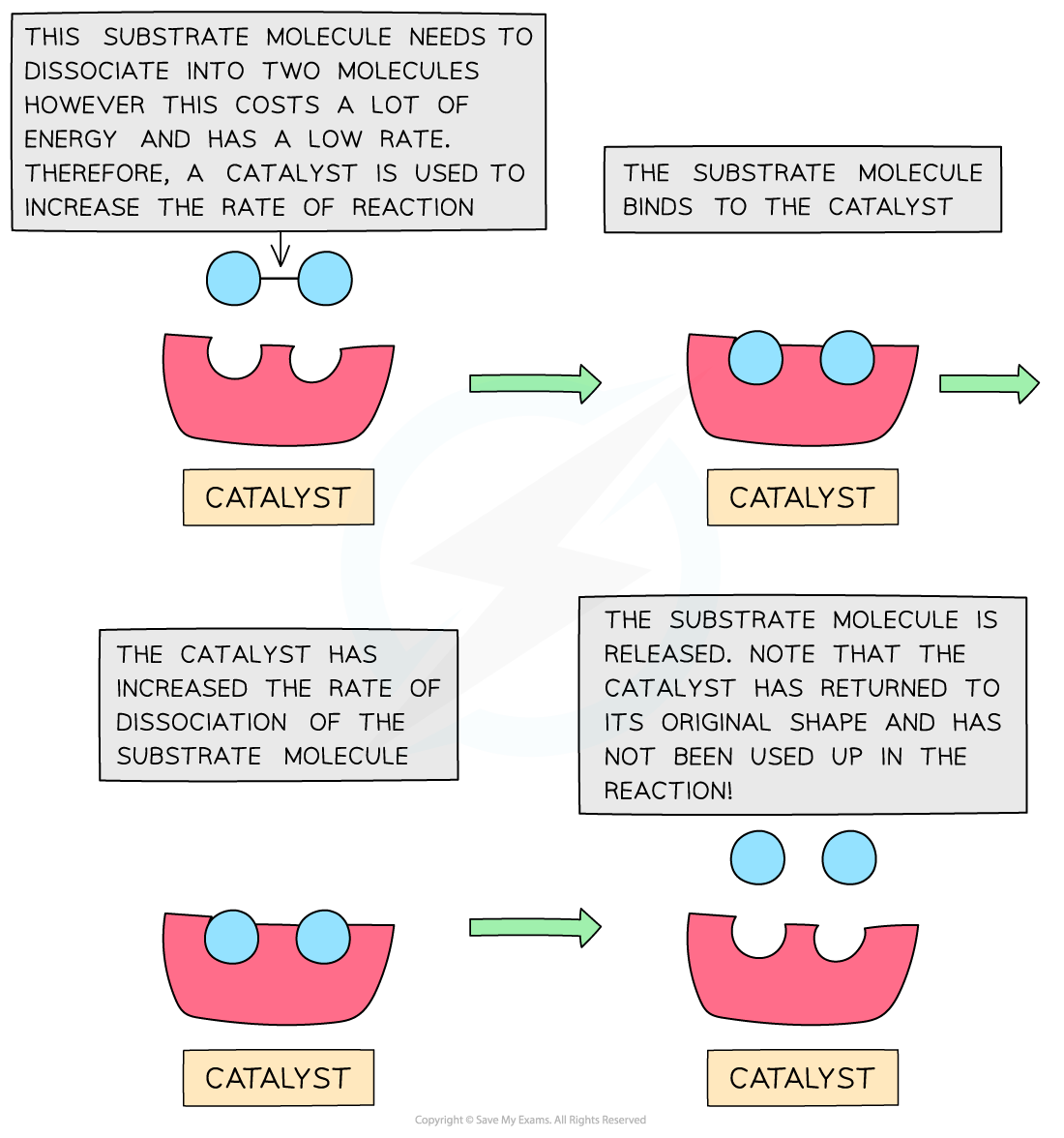
The diagram shows that the catalyst speeds up a reaction that would normally be slow due to the high activation energy. The catalyst is not used up in the chemical reaction and is not taking part in the chemical reaction
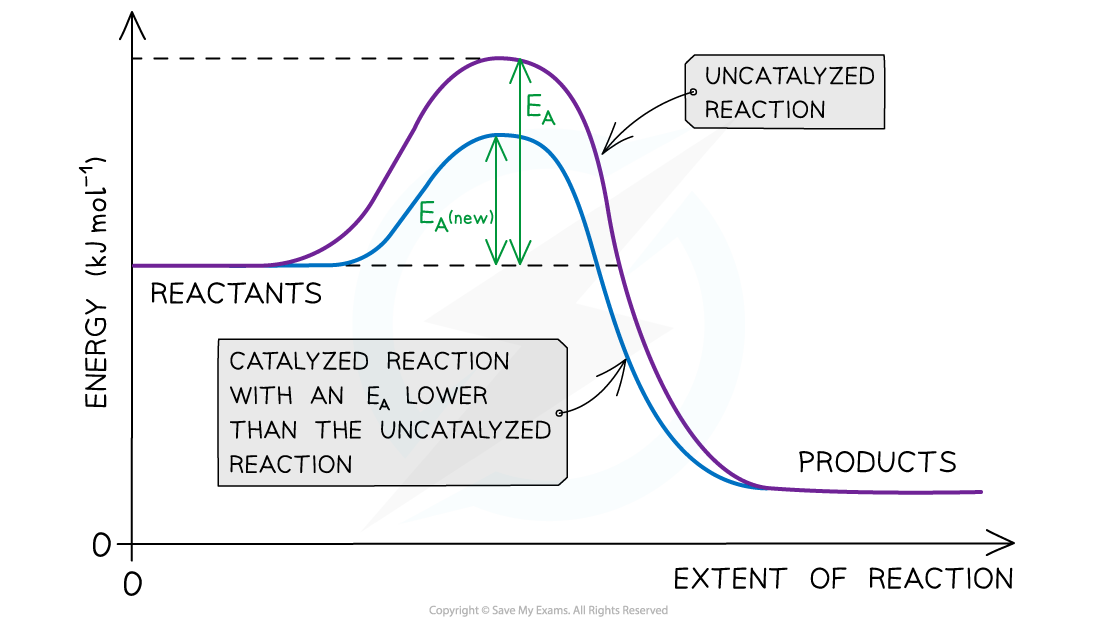
The diagram shows that the catalyst allows the reaction to take place through a different mechanism, which has a lower activation energy than the original reaction
Worked Example
The energy profile below shows the energy changes for a reaction with and without a catalyst.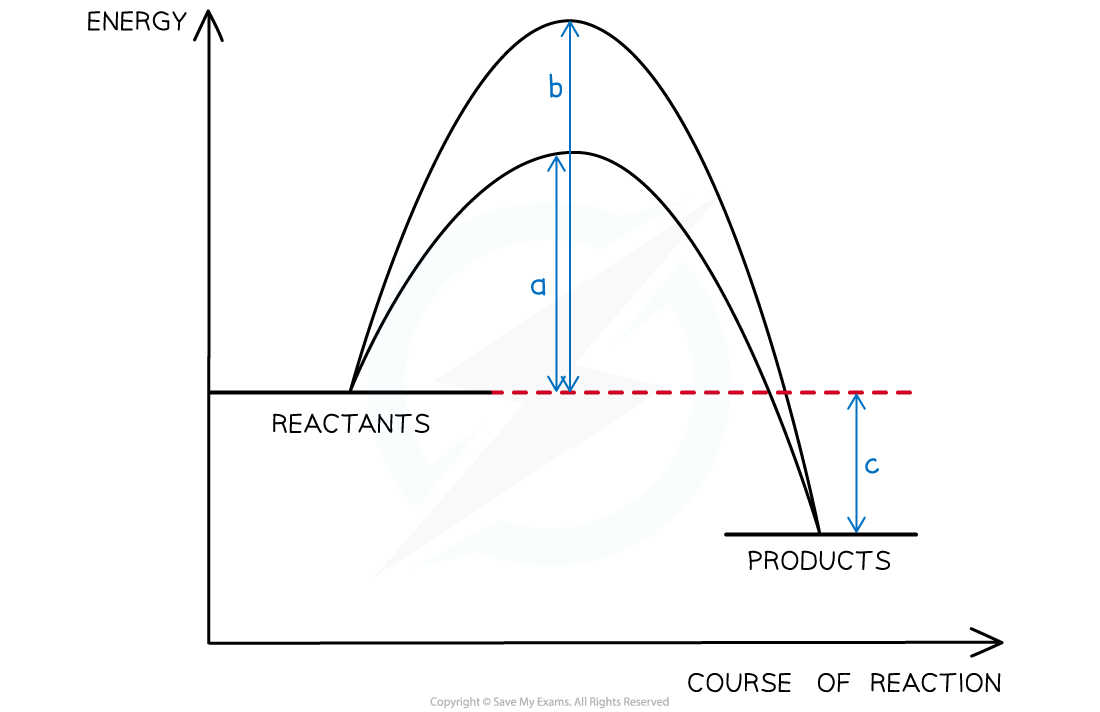 Which symbols represent the enthalpy change, ∆H , and the activation energy, Ea , for the reaction using a catalyst?
Which symbols represent the enthalpy change, ∆H , and the activation energy, Ea , for the reaction using a catalyst?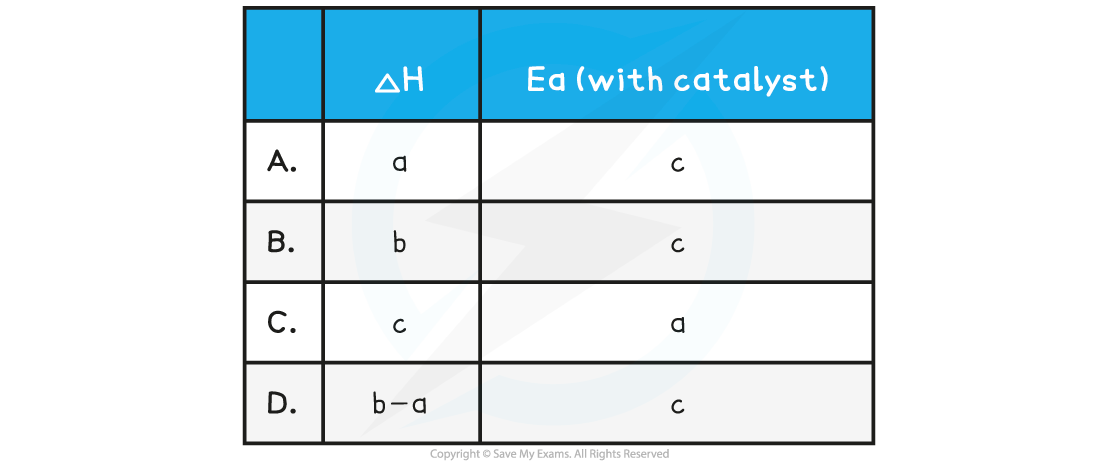
Answer:
The correct option is C.
-
- By definition the enthalpy change is the difference in energy content between reactants and products, in this case, arrow c
- The catalyst lowers the activation energy, so that corresponds to arrow a
转载自savemyexams

在线登记
最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








