- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记6.1.4 Activation Energy
Activation Energy
- For a reaction to take place, the reactant particles need to overcome a minimum amount of energy
- This energy is called the activation energy (Ea)
- In exothermic reactions the reactants are higher in energy than the products
- In endothermic reactions the reactants are lower in energy than the products
- Therefore, the Ea in endothermic reactions is relatively larger than in exothermic reaction
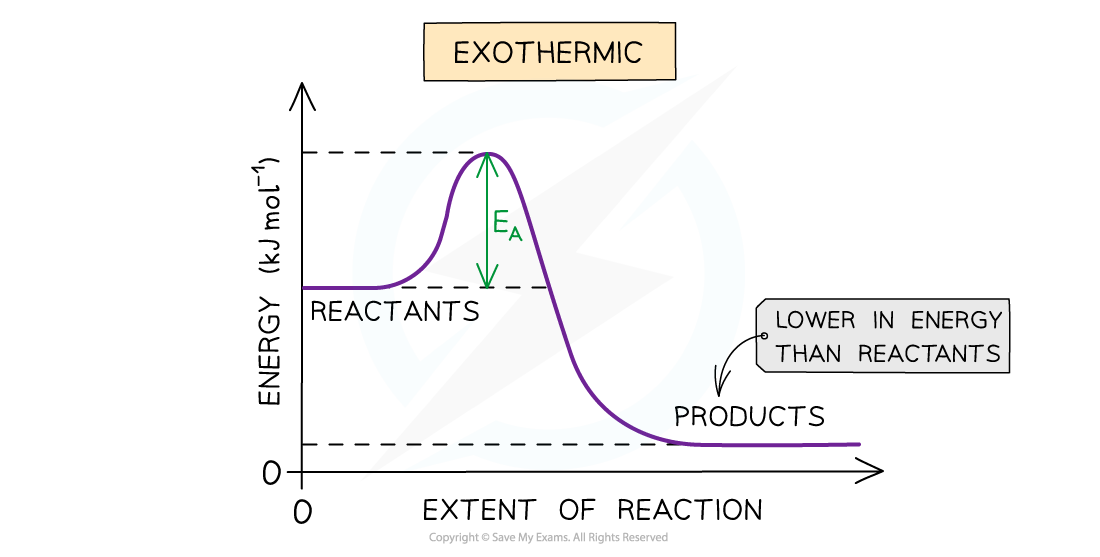
The diagram shows that the reactants are higher in energy than the products in the exothermic reaction, so the energy needed for the reactants to go over the energy barrier is relatively small
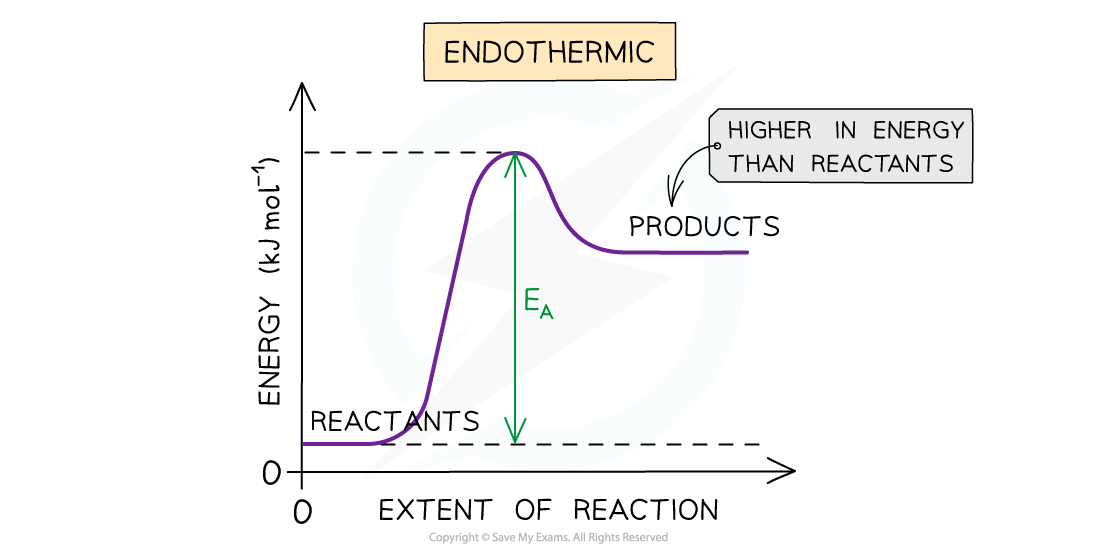
The diagram shows that the reactants are lower in energy than the products in the endothermic reaction, so the energy needed for the reactants to go over the energy barrier is relatively large
- Even though particles collide with each other in the same orientation, if they don’t possess a minimum energy that corresponds to the Ea of that reaction, the reaction will not take place
- Therefore, for a collision to be successful the reactant particles must collide in the correct orientation AND possess a minimum energy equal to the Ea of that reaction
Exam Tip
You may be required to show or calculate the activation energy for a reverse reaction using a labelled energy profile like those above. The activation energy for the reverse reaction is found by:
For an exothermic reaction = ∆H + Ea (forward)
For an endothermic reaction = Ea (forward) -∆H
Calculations of the value of the activation energy from experimental data are not required at Standard Level but are part of Topic 16 in Higher Level Chemistry
How catalysts work
By decreasing Ea, a catalyst increases the rate of a chemical reaction, without itself being permanently chemically changed.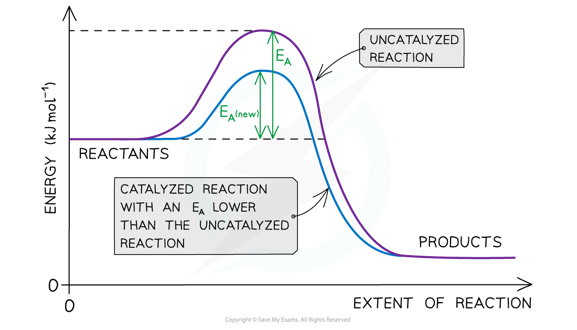
- More particles are able to collide with sufficient energy to react under the lower activation energy
- More frequent, successful collisions lead to a faster rate of reaction
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









