- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记6.1.2 Rate of Reaction
Rate of Reaction
Reaction rate
- Some reactions take place instantly, but most are much slower and it is possible to measure how long these reactions take to reach a certain stage
- As a chemical reaction proceeds, the concentration of the reactants decreases and the concentration of the products increases
- The rate of a reaction is the speed at which a chemical reaction takes place and has units mol dm-3 s-1
- The rate of a reaction can be calculated by:

- Graphically we can represent the rate of reaction as:

Rate of reaction graphs
Worked Example
Iodine and methanoic acid react in aqueous solution.
I2 (aq) + HCOOH (aq) → 2I− (aq) + 2H+ (aq) + CO2 (g)
The rate of reaction can be found by measuring the volume of carbon dioxide produced per unit time and plotting a graph as shown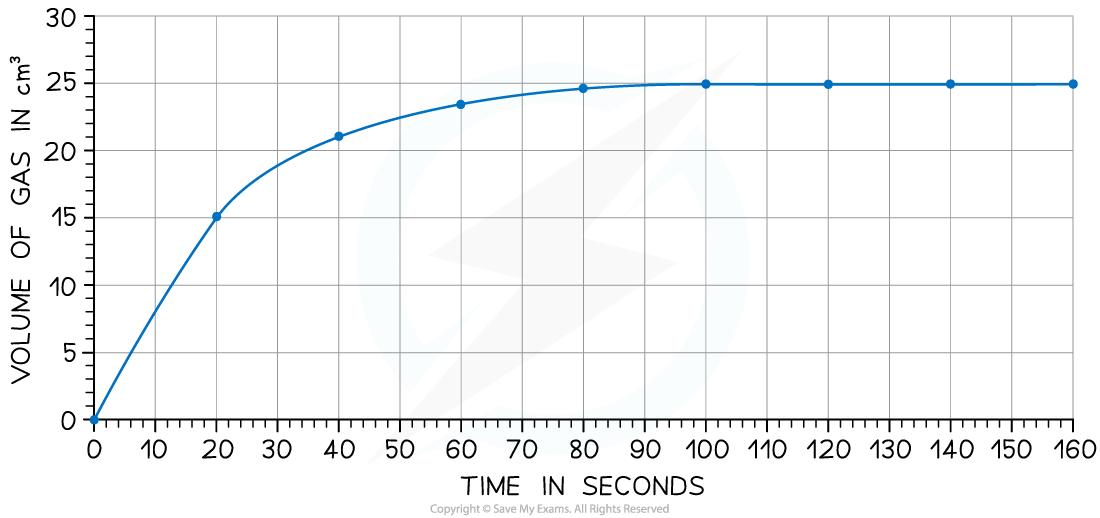 Calculate the rate of reaction at 20 seconds
Calculate the rate of reaction at 20 seconds
Answer:
-
- Draw a tangent to the curve at 20 seconds:
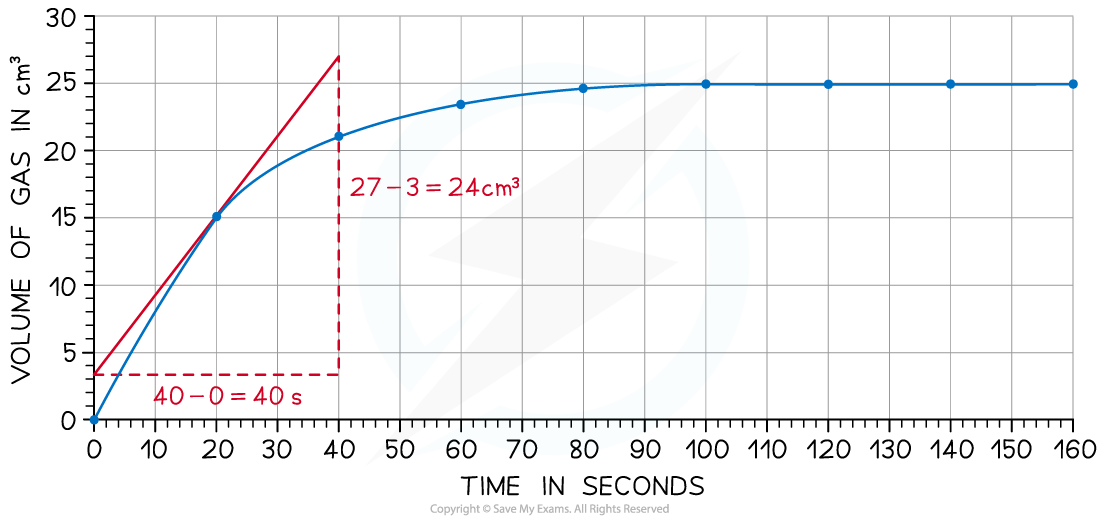
-
- Complete the triangle and read off the values of x and y
- Determine the gradient of the line using ∆y / ∆x
- Rate of reaction = 24 ÷ 40 = 0.60 cm3 s-1
Exam Tip
When drawing the tangent to a curve make the triangle large and try to intersect with gridlines if you can. This minimises errors of precision and reduces the chance you will accidently misread the graph values
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








