- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记5.3.1 Bond Enthalpy
Bonds & Energy
- When bonds are broken or made enthalpy changes take place
- A chemical bond is a force of attraction between two atoms
- Breaking the bond requires the input of energy it is therefore an endothermic process
- The energy change required to break the bond depends on the atoms that form the bond
- The energy required to break a particular bond is called the bond dissociation enthalpy
- This is usually just shortened to bond enthalpy or bond energy
- Bond formation is the opposite of bond breaking and so energy is released when bonds are formed
- It is therefore an exothermic process
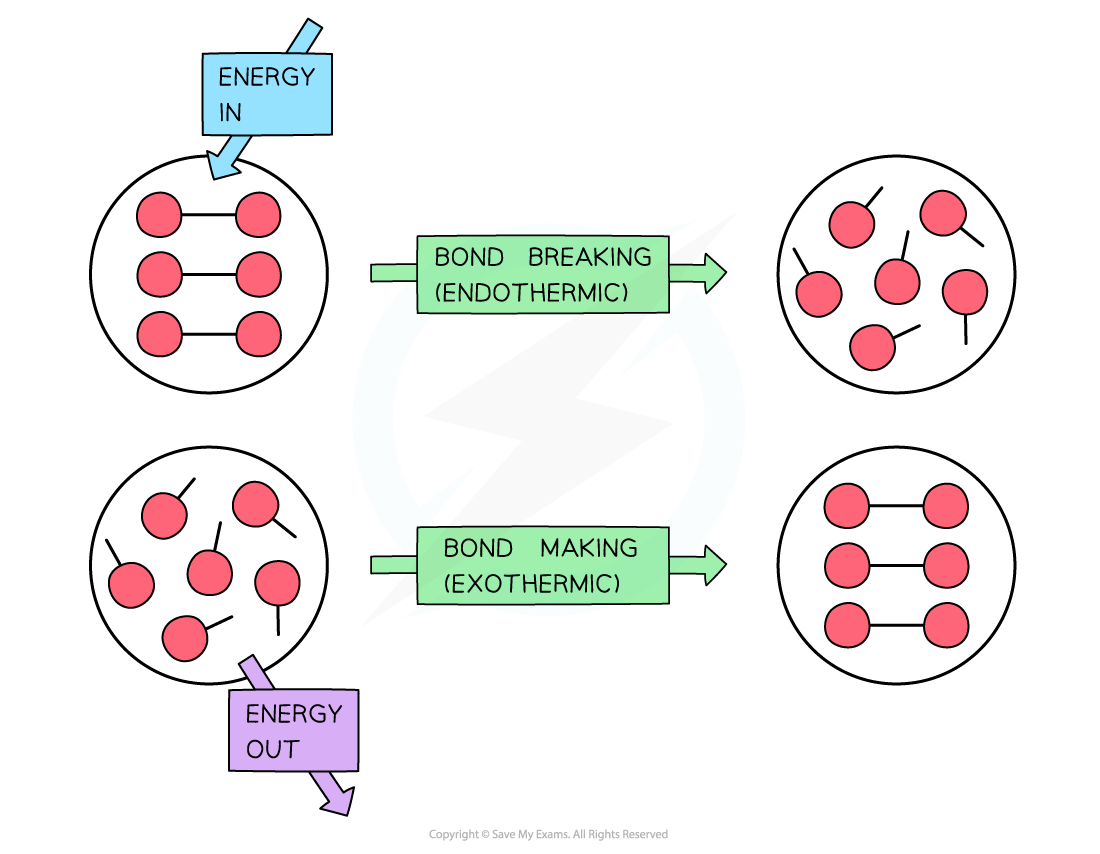
To break bonds energy is required from the surroundings and to make new bonds energy is released from the reaction to the surroundings
- The amount of energy released when a particular bond is formed has the same magnitude as the energy taken in when the bond is broken but has the opposite sign
Overall enthalpy changes
- If more energy is released when new bonds are formed than energy is required to break bonds, the reaction is exothermic
- The products are more stable than the reactants
- If more energy is required to break bonds than energy is released when new bonds are formed, the reaction is endothermic
- The products are less stable than the reactants
- The relationship between bond breaking and bond making can be shown graphically like this:
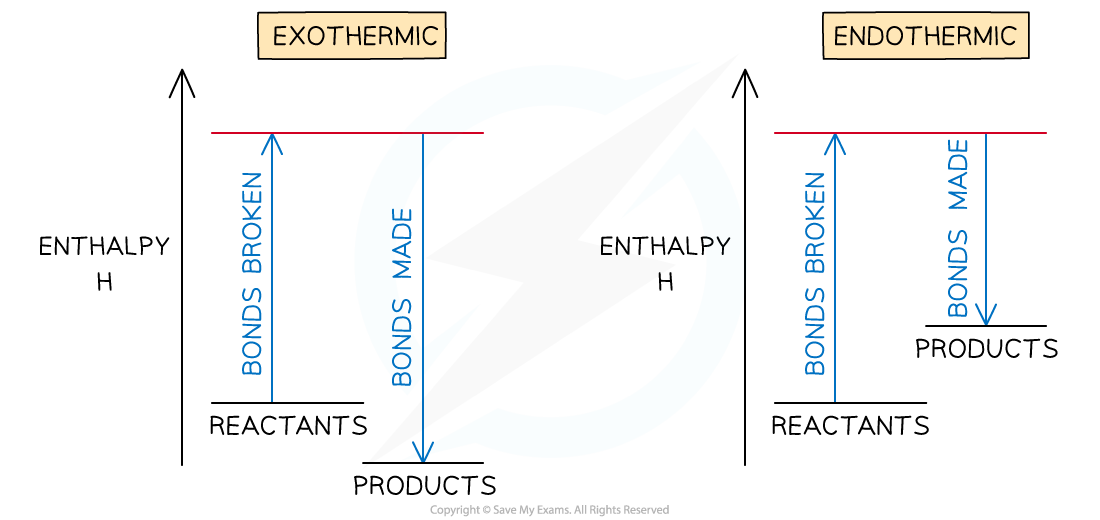
Bond enthalpy profiles
Bond Enthalpy
Average bond energy
- Bond energies are affected by other atoms in the molecule (the environment)
- Therefore, an average of a number of the same type of bond but in different environments is calculated
- This bond energy is known as the average bond energy and is defined as
'The energy needed to break one mole of bonds in a gaseous molecule averaged over similar compounds'
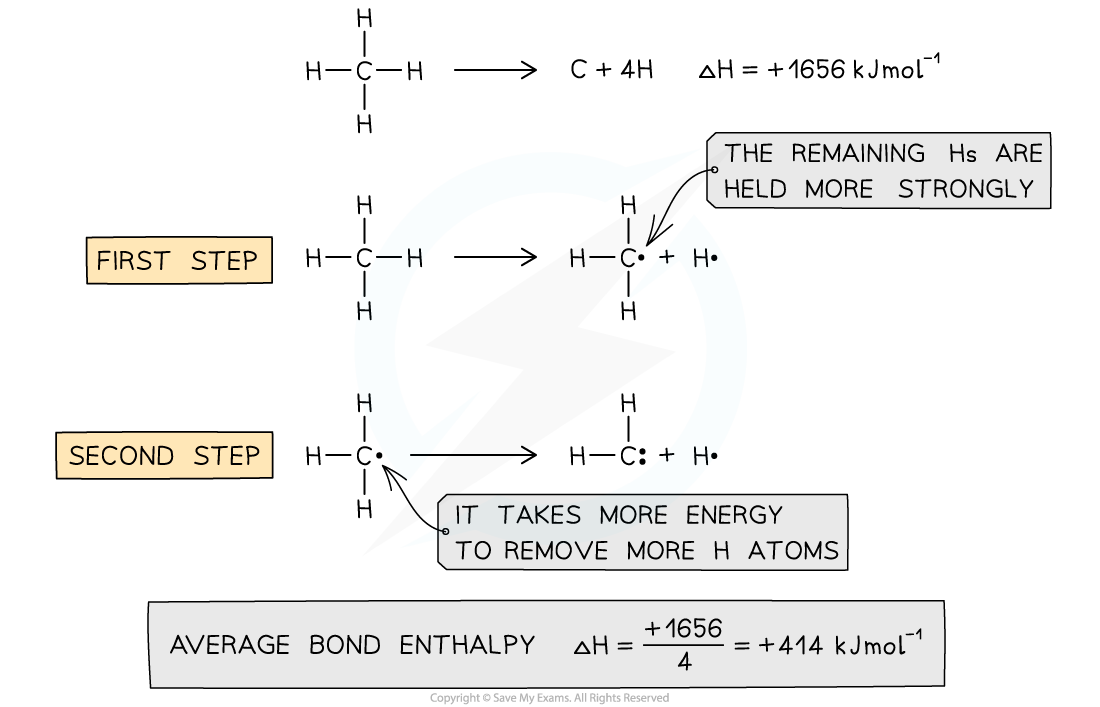
Average bond enthalpy of C-H in methane
- The average bond enthalpy of C-H is found by taking the bond dissociation enthalpy for the whole molecule and dividing it by the number of C-H bonds
- The first C-H bond is easier to break than the second as the remaining hydrogens are pulled more closely to the carbon
- However, since it is impossible to measure the energy of each C-H bond an average is taken
- This value is also compared with a range of similar compounds to obtain an accepted value for the average bond enthalpy
Exam Tip
A lot of students mix up endothermic / exothermic and bond breaking / bond making.
An easy way to remember is that ENDOTHERMIC leads to the poetic phrase the 'end o' the bond'
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








