- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记4.2.3 Predicting Molecular Shapes
Predicting Shapes & Bond Angles
- Before you predict the shape of any molecule work out the Lewis structure to determine the number of bonding and lone pairs
- Apply the VSEPR rules and you should be successful in deducing the correct shape and bond angle
Worked Example
Predict the domain geometry, shape and bond angle in the following molecules or ions:
- H2S
- NH2Cl
- NO2+
- ClF2+
Answers:
Answer 1: The total number of valence electrons in H2S is = 1 + 1 + 6 = 8, so there are four pairs of electrons around S
Hydrogen only forms one bond, so there are two bonding pairs and two lone pairs: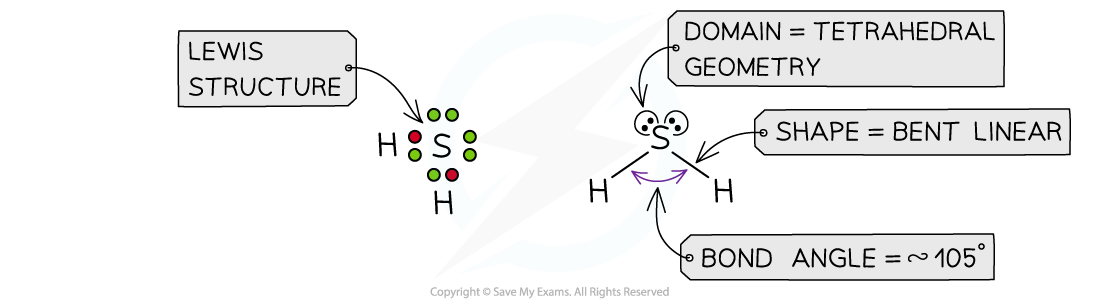
Answer 2: The total number of valence electrons in NH2Cl is = 5 + 1 + 1 + 7 = 14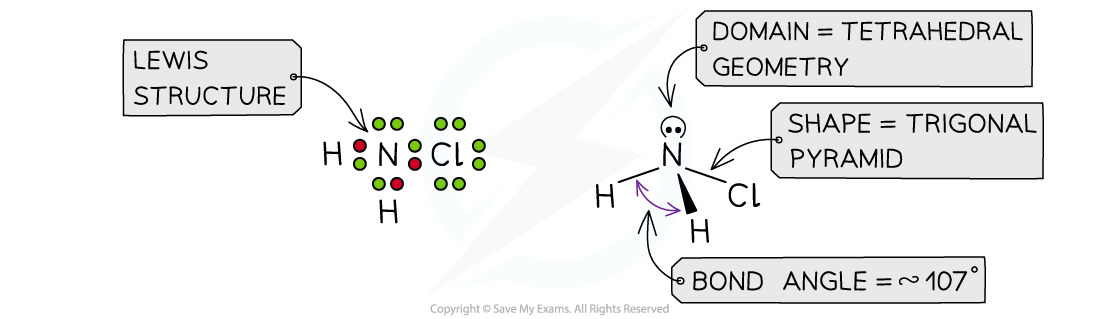
Answer 3: The total number of valence electrons in NO2+= 5 + 6 + 6 -1 = 16 (subtracting one for the positive charge)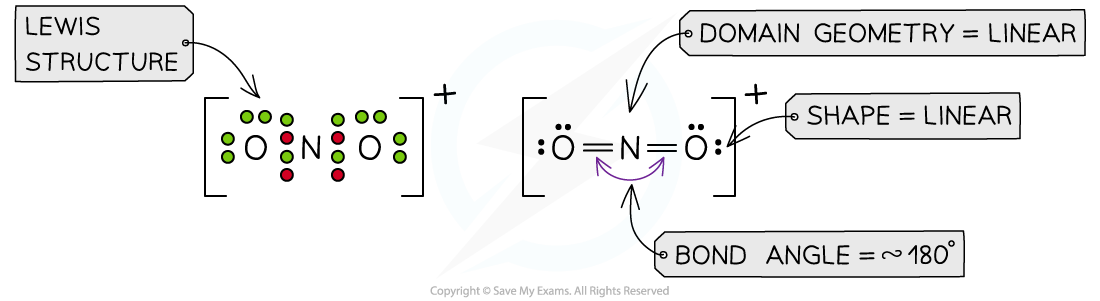
Answer 4: The total number of valence electrons in ClF2+= 7 + 7 + 7 -1 = 20 (subtracting one for the positive charge)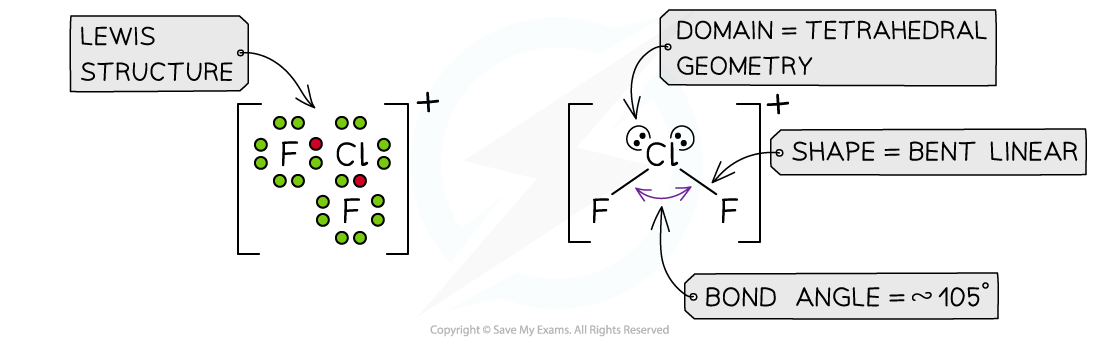
Exam Tip
For Standard Level Chemistry you are only required to know the shape of molecules up to four electron domains.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









