- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记4.1.5 Bond Polarity
Bond Polarity
- When two atoms in a covalent bond have the same electronegativity the covalent bond is nonpolar
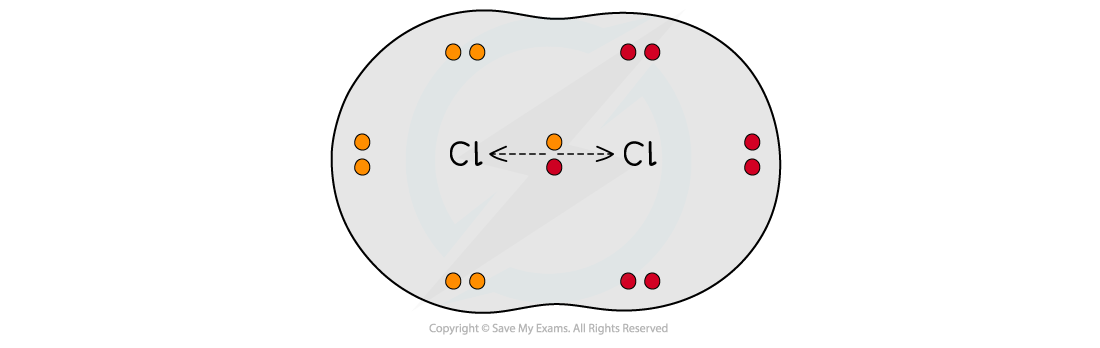
The two chlorine atoms have identical electronegativities so the bonding electrons are shared equally between the two atoms
- When two atoms in a covalent bond have different electronegativities the covalent bond is polar and the electrons will be drawn towards the more electronegative atom
- As a result of this:
- The negative charge centre and positive charge centre do not coincide with each other
- This means that the electron distribution is asymmetric
- The less electronegative atom gets a partial charge of δ+ (delta positive)
- The more electronegative atom gets a partial charge of δ- (delta negative)
- The greater the difference in electronegativity the more polar the bond becomes
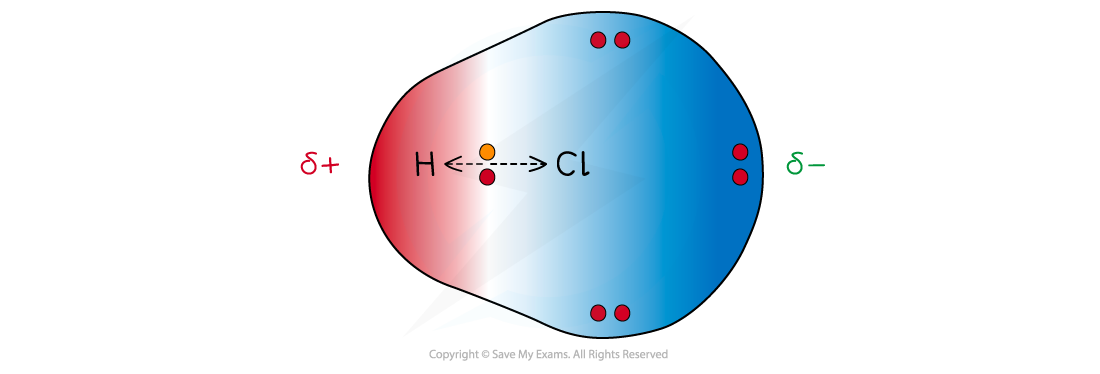
Cl has a greater electronegativity than H causing the electrons to be more attracted towards the Cl atom which becomes delta negative and the H delta positive
Dipole moment
- The dipole moment is a measure of how polar a bond is
- The direction of the dipole moment is shown by the following sign in which the arrow points to the partially negatively charged end of the dipole:

The sign shows the direction of the dipole moment and the arrow points to the delta negative end of the dipole
Worked Example
The electronegativity values of four elements are given.
C = 2.6 N = 3.0 O = 3.4 F = 4.0
What is the order of increasing polarity of the bonds in the following compounds?A. CO < OF2 < NO < CF4
B. NO < OF2 < CO < CF4
C. CF4 < CO < OF2 < NO
D. CF4 < NO < OF2 < CO
Answer:
The correct option is B.
-
- You have to calculate the difference in electronegativity for the bonds and then rank them from smallest to largest:
NO (3.4 - 3.0 = 0.4)
OF2 (4.0 - 3.4 = 0.6)
CO (3.4 - 2.6 = 0.8)
CF4 (4.0 - 2.6 = 1.4)
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









