- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: HL复习笔记2.1.6 Energy Levels & Sublevels
Electron Energy Levels
Shells
- The arrangement of electrons in an atom is called the electronic configuration
- Electrons are arranged around the nucleus in principal energy levels or principal quantum shells
- Principal quantum numbers (n) are used to number the energy levels or quantum shells
- The lower the principal quantum number, the closer the shell is to the nucleus
- The higher the principal quantum number, the lesser the energy of the shell
- Each principal quantum number has a fixed number of electrons it can hold
- n = 1 : up to 2 electrons
- n = 2 : up to 8 electrons
- n = 3 : up to 18 electrons
- n = 4 : up to 32 electrons
- There is a pattern here - the mathematical relationship between the number of electrons and the principal energy level is 2n2
- So for example, in the third shell n = 3 and the number of electrons is 2 x (32 ) = 18
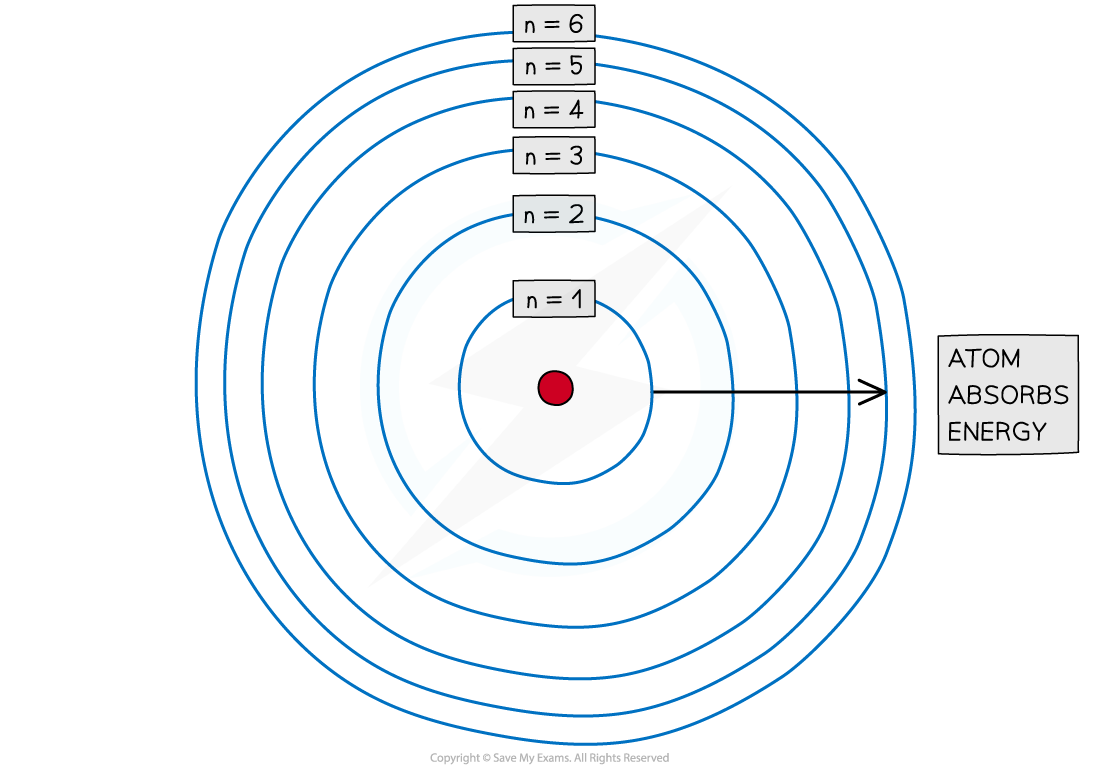
Electrons are arranged in principal quantum shells, which are numbered by principal quantum numbers
Subshells
- The principal quantum shells are split into subshells which are given the letters s, p and d
- Elements with more than 57 electrons also have an f subshell
- The energy of the electrons in the subshells increases in the order s < p < d
- The order of subshells overlap for the higher principal quantum shells as seen in the diagram below:
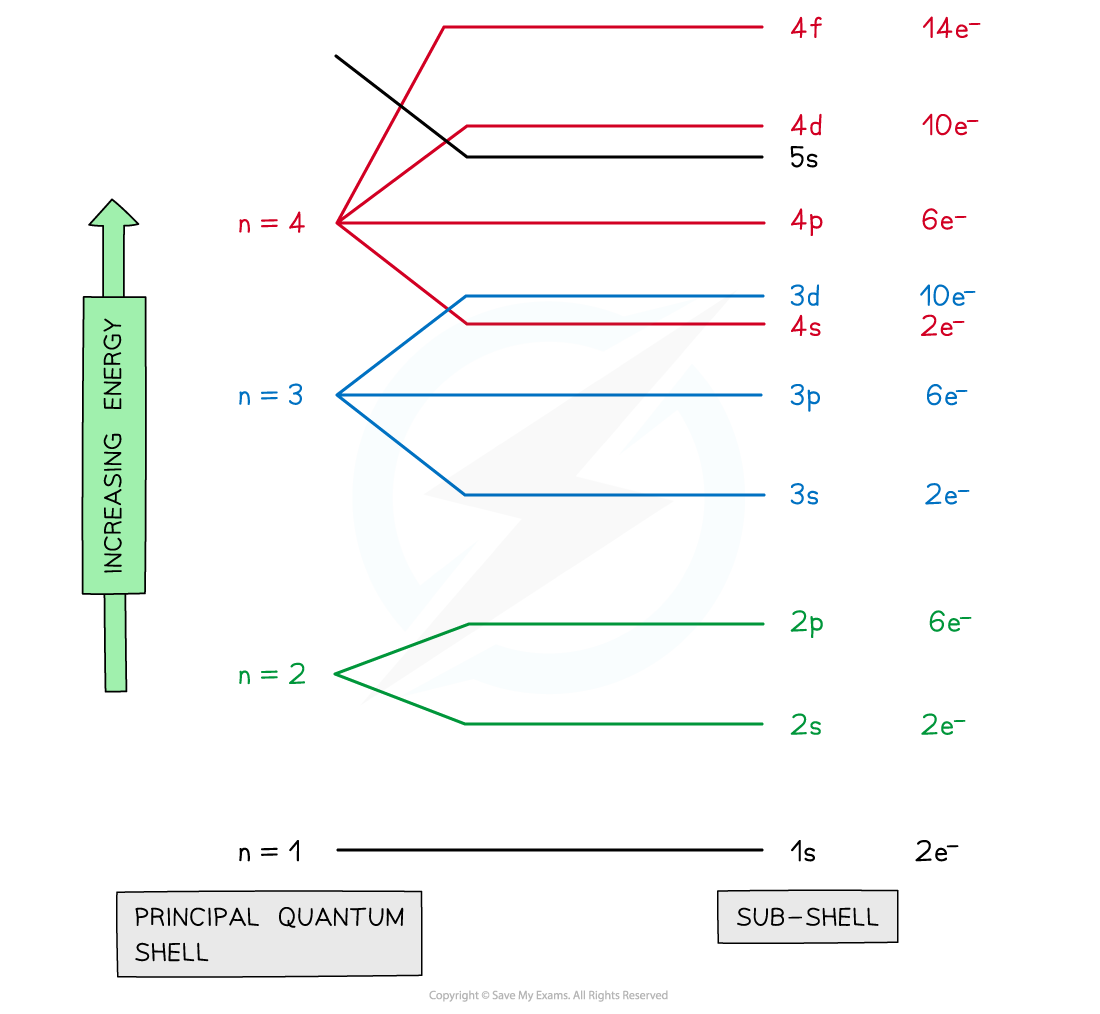
Electrons are arranged in principal quantum shells, which are numbered by principal quantum numbers
Orbitals
- The subshells contain one or more atomic orbitals
- Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between
- Each atomic orbital can be occupied by a maximum of two electrons
- The orbitals have specific 3D shapes
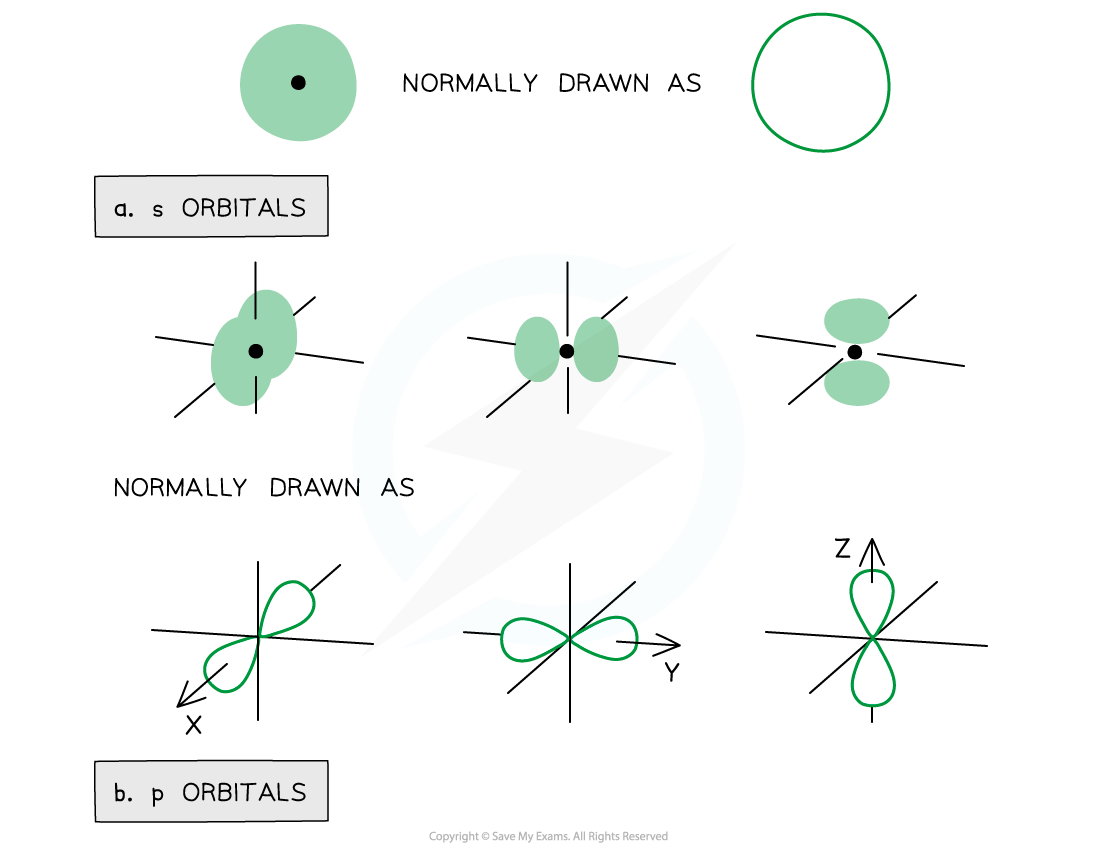
Representation of orbitals (the dot represents the nucleus of the atom) showing spherical s orbitals (a), p orbitals containing ‘lobes’ along the x, y and z axis
- Note that the shape of the d orbitals is not required for IB Chemistry
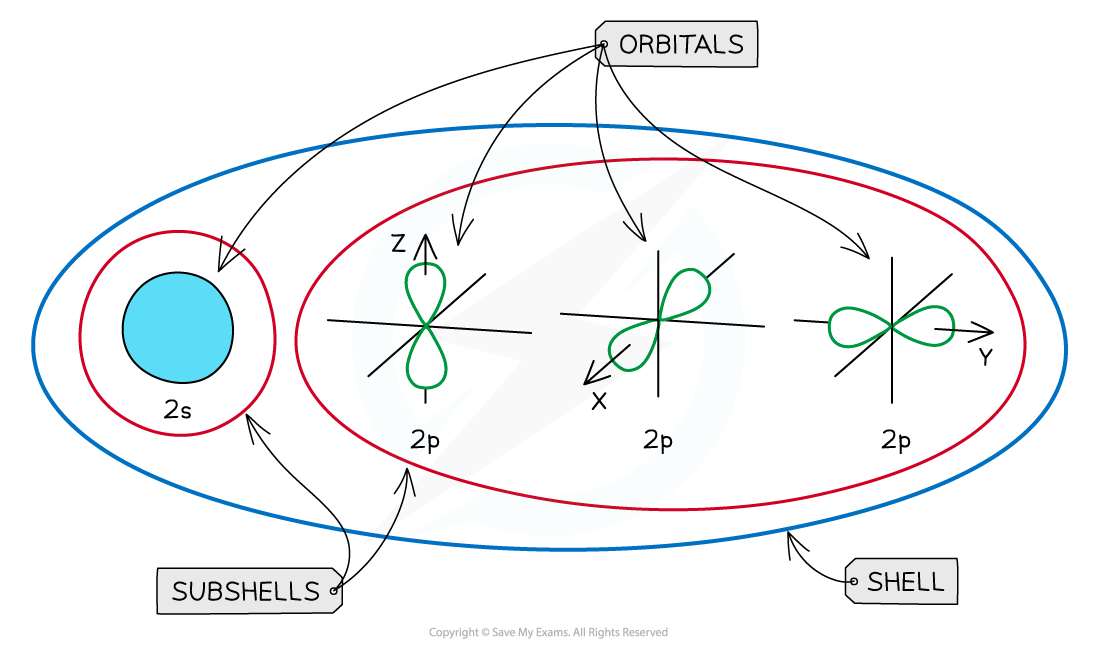
An overview of the shells, subshells and orbitals in an atom
Ground state
- The ground state is the most stable electronic configuration of an atom which has the lowest amount of energy
- This is achieved by filling the subshells of energy with the lowest energy first (1s) - this is called the Aufbau Principle
- The order of the subshells in terms of increasing energy does not follow a regular pattern at n= 3 and higher
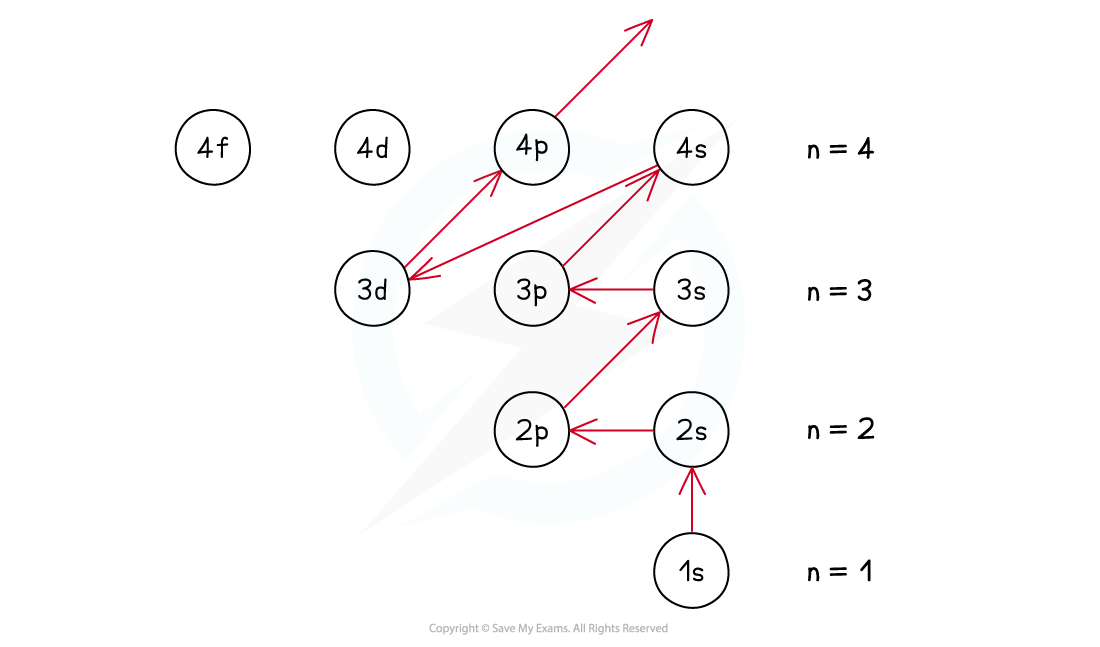
The Aufbau Principle - following the arrows gives you the filling order
Sublevels & Energy
- The principal quantum shells increase in energy with increasing principal quantum number
- Eg. n = 4 is higher in energy than n = 2
- The subshells increase in energy as follows: s < p < d < f
- The only exception to these rules is the 3d orbital which has slightly higher energy than the 4s orbital, so the 4s orbital is filled before the 3d orbital
- All the orbitals in the same subshell have the same energy and are said to be degenerate
- Eg. px, py and pz are all equal in energy
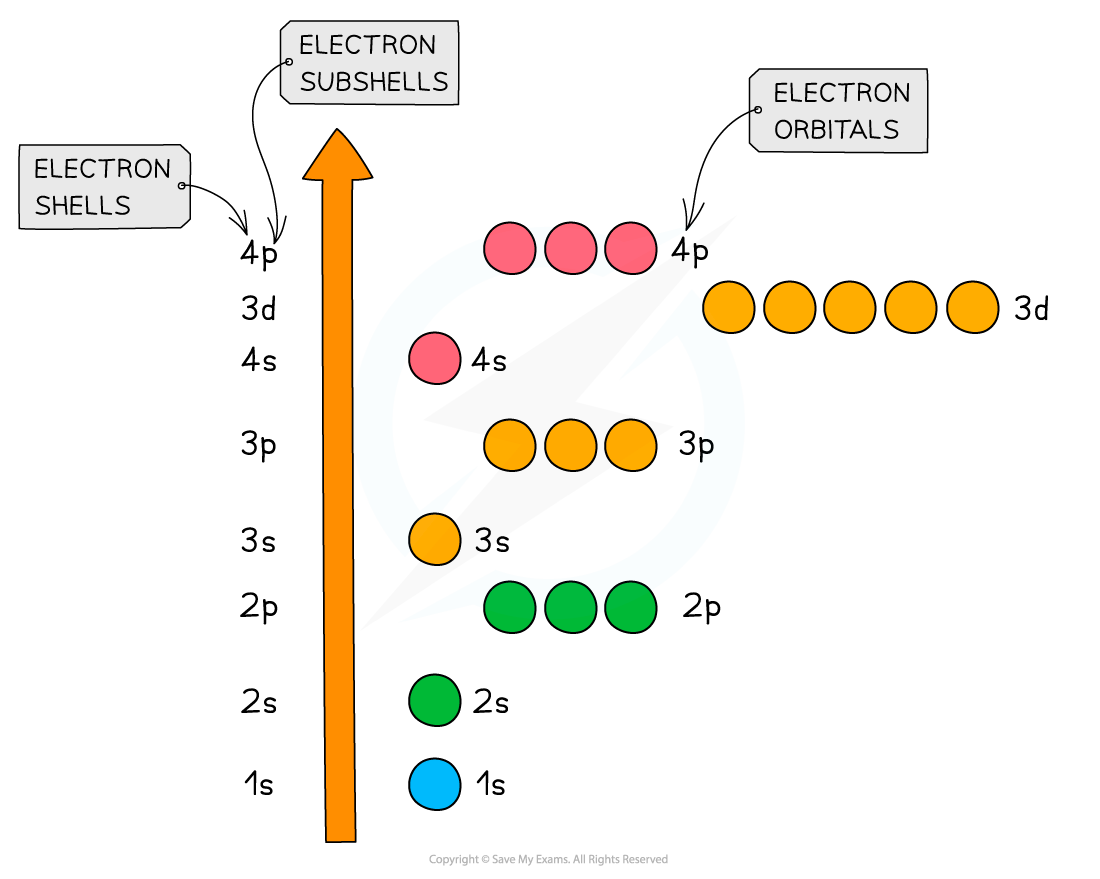
Relative energies of the shells and subshells
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








