- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Biology: HL复习笔记8.1.2 Inhibition
Enzyme Inhibitors
- Inhibitors are chemical substances that can bind to an enzyme and reduce its activity
- Inhibitors can be formed from within the cell or can be introduced from the external environment
- An enzyme's activity can be reduced or stopped, temporarily, by an inhibitor
- There are two types of inhibitors: competitive and non-competitive
Competitive inhibitors
- Competitive inhibitors have a similar shape to that of the substrate molecules
- They bind to the active site of the enzyme, interfering with it and competing with the substrate for the active site
- The substrate, therefore, cannot bind to the active site if a competitive inhibitor is already bound
Non-competitive inhibitors
- Non-competitive inhibitors bind to the enzyme at an alternative site, which alters the shape of the active site
- This therefore prevents the substrate from binding to the active site
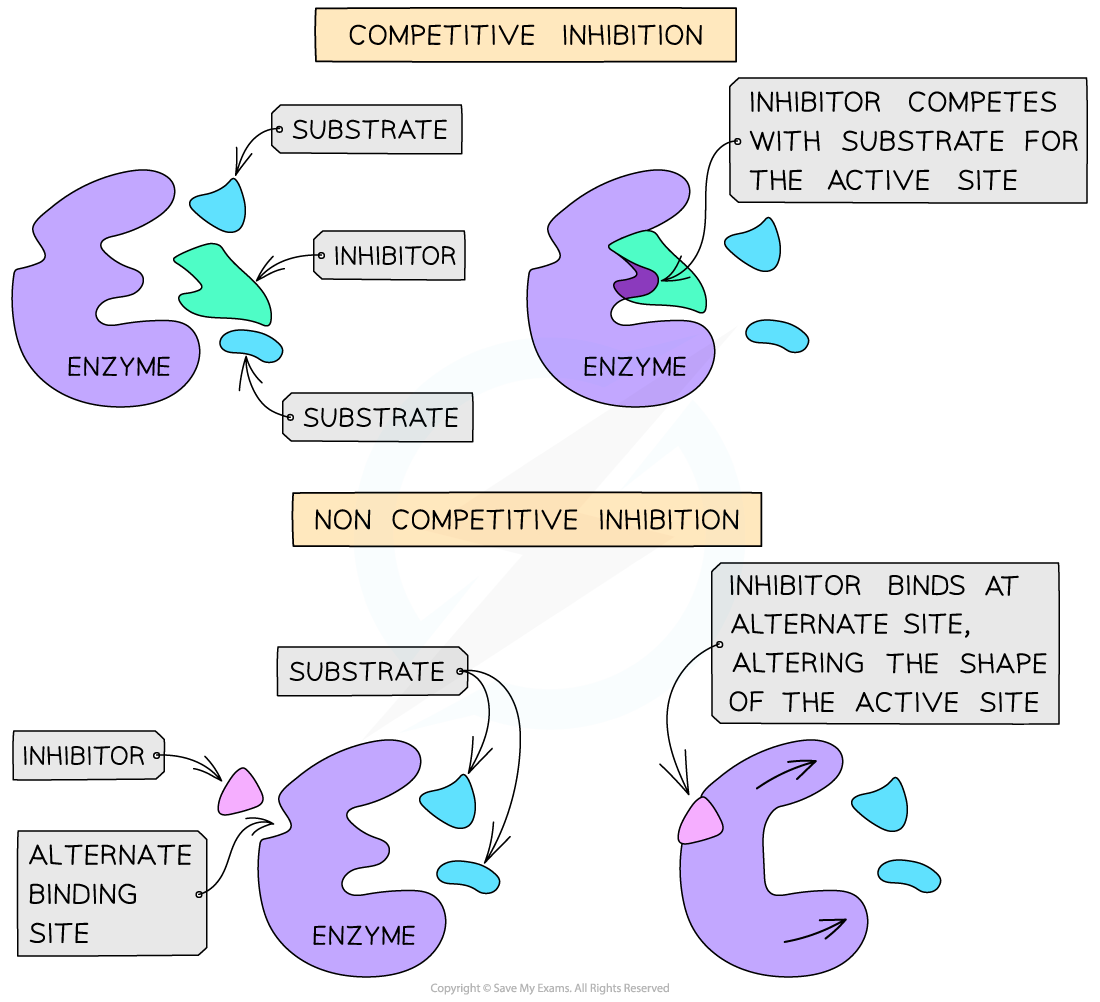
Competitive and non-competitive inhibition
Examples of competitive and non-competitive inhibitors
- An example of a competitive inhibitor involves the enzyme RuBisCo, an important carbon fixation enzyme in photosynthesis
- Oxygen is a competitive inhibitor to this enzyme and blocks the active site for carbon dioxide
- Therefore carbon dioxide cannot bind to RuBisCo and reactions involved in photosynthesis slow down or cease to occur
- This can be fatal to the plant
- An example of a non-competitive inhibitor involves the enzyme cytochrome c oxidase, a mitochondrial enzyme that catalyses one of the key reactions in aerobic respiration
- Cyanide ions are a non-competitive inhibitor that binds to a site on the enzyme and change the shape of the active site
- Therefore cytochrome c oxidase cannot carry out its functions in respiration
- This can be fatal as it takes too long to produce new enzymes and the organism will die before this can occur
- Cyanide is known as a metabolic poison because it interferes with metabolic pathways
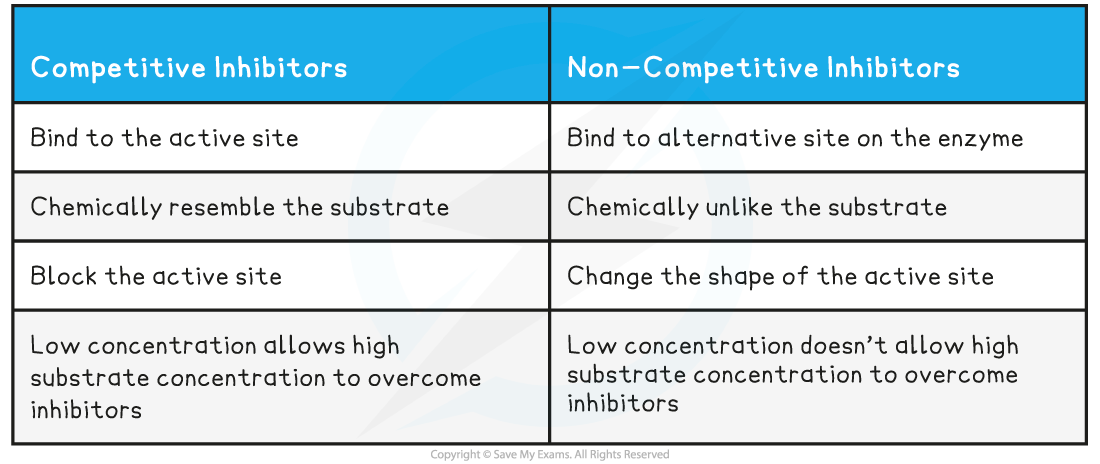
Table comparing competitive and non-competitive inhibitors
Exam Tip
You need to be able to give a named example for competitive and non-competitive inhibition
End-product Inhibition
- Enzymes can be regulated by chemical substances that bind to a site on the enzyme away from the active site, known as the allosteric site
- Binding to this site, away from the active site forms an allosteric interaction leading to a reversible change in the shape and activity
- Chemicals that regulate the metabolic pathway like this are termed allosteric regulators
- End-product inhibition occurs when the end product from a reaction is present in excess and itself acts as a non-competitive inhibitor of the enzyme
- The end product binds to an allosteric site on the enzyme and causes inhibition of the pathway, so they are referred to as allosteric inhibitors
- Allosteric inhibitors are important to prevent the build-up of intermediate products in a metabolic pathway, as each small step of the pathway may produce a new product
- The product therefore does not accumulate and the pathway can continue
- An outline of the process is as follows:
- As the enzyme converts substrate to an end product, the process is itself slowed down as the end-product of the reaction chain binds to an allosteric site on the original enzyme, changing the shape of the active site and preventing the formation of further enzyme-substrate complexes
- The inhibition of the enzyme means that product levels fall, at which point the enzyme begins catalysing the reaction once again; this is a continuous feedback loop
- The end-product inhibitor eventually detaches from the enzyme to be used elsewhere; this is what allows the active site to reform and the enzyme to return to an active state
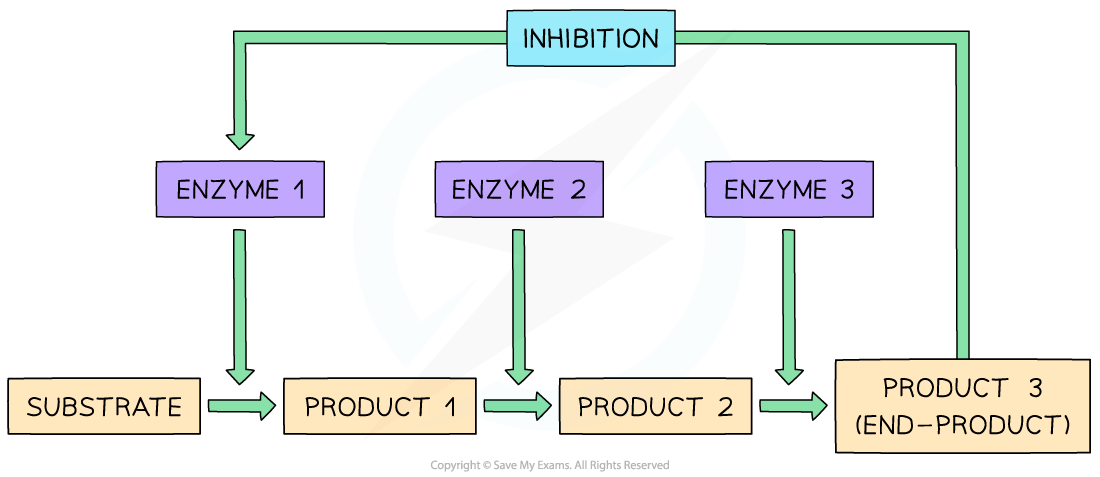 End-product inhibition where the end-product of an enzyme controlled pathway inhibits the starting enzyme and limits the reactions
End-product inhibition where the end-product of an enzyme controlled pathway inhibits the starting enzyme and limits the reactions
- The end-product inhibitor eventually detaches from the enzyme to be used elsewhere; this is what allows the active site to reform and the enzyme to return to an active state
- An outline of the process is as follows:
Worked Example
Show, with a diagram, the end-product inhibition of the pathway that converts threonine to isoleucine
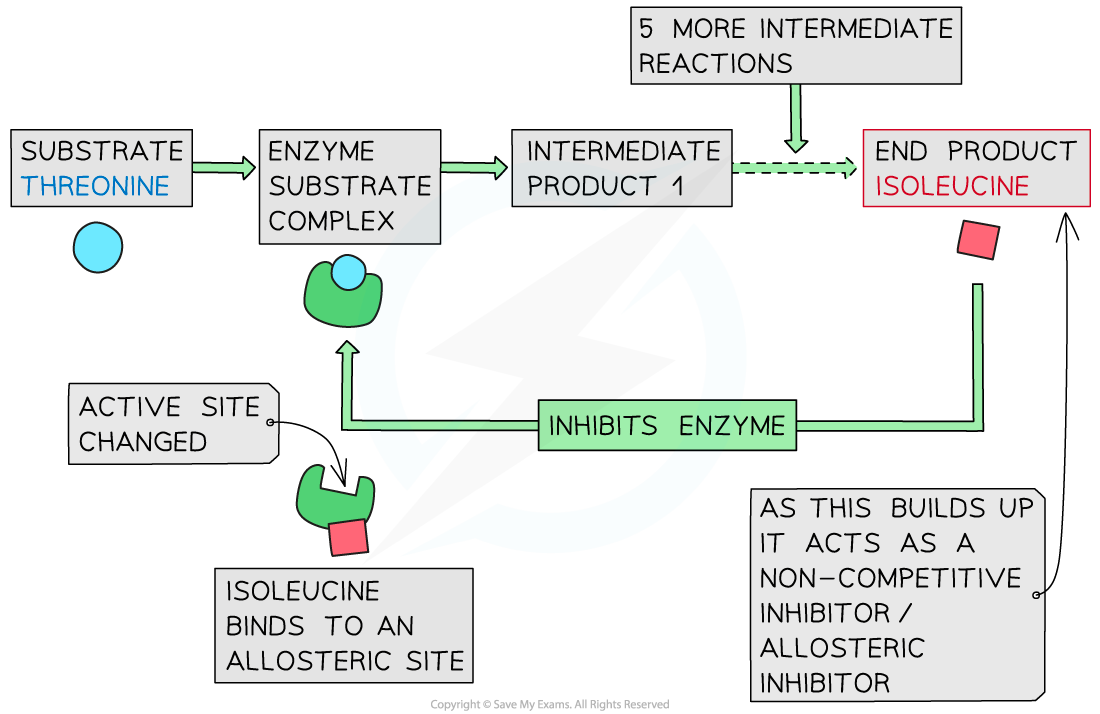
Example of end-product inhibition between threonine and isoleucine
Exam Tip
You need to know the specific example of end-product inhibition of theonine and isoleucine
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









